Getting Started
Please review Using the Facility before making an appointment.
New users must attend the Intro to Flow Cytometry lecture series before signing up for any training on any cytometer. Training on the microscope does not require the lecture series.
After attending the lecture series, make an appointment in iLab for 1.5 hours on the instrument(s) to be used. New users will have access to only the specified Assisted/Training times listed below until training is completed. Please contact the Flow Lab at uwflow@uwcarbone.wisc.edu if you need to schedule Assisted/Training outside specified times.
Jump to:
Equipment
- Cytek Auroras
- ThermoFisher Attunes
- BD LSR Fortessa
- ImageStream
- BD FACSAriaIII Cell Sorter "Jayne"
- BD FACSDiscover S8 Cell Sorter
- Sony MA900 Cell Sorter
- ThermoFisher Countess 3 FL Automated Cell Counter
- Cell Counter: Nexcelom Cellometer
- Analysis Computers
Cytek Aurora Spectral Flow Cytometers

Location: WIMR 7018A (Pinky), and 7018D (Clyde)
The Cytek Aurora is a 5 Laser Spectral Cytometer, capable analyzing 40+ color cytometry samples. These instruments captures the bulk of the emission spectra of each fluorochrome, allowing for fluorochrome combinations not possible on traditional flow cytometers, thus increasing the number of fluorochromes possible per panel. These instruments are matched and are equipped with 5 lasers (355nm UV, 405nm Violet, 488nm Blue, 561nm Yellow-Green, and 640nm Red). It has three light scatter channels, Forward Scatter and Side Scatter off both the Blue and Violet Lasers, and 64 fluorescence channels. Samples can be acquired from traditional 12x75mm flow tubes as well as from 96 well plates). Instruments are named “Pinky” and “Clyde.”
There are some minor considerations for running ongoing assays on both instruments. Contact the Flow Lab at uwflow@uwcarbone.wisc.edu for a FREE and short consult before booking.
Contact the Flow Lab to set up a personalized consultation or training session at uwflow@uwcarbone.wisc.edu
ThermoFisher Attune CytPix V6 Flow Cytometer: “Tempest”
Location: WIMR 7018D
The Attune CytPix is a benchtop analysis cytometer with 4 lasers (488, 405, 561, and 633nm) and a brightfield camera. Capable of collecting up to 16 parameters: 2 scatter and 14 fluorescent, the optical bench is user-configurable to accommodate unusual fluorochromes and unique combinations. The V6 model is configured with 6 channels off the violet laser, allowing expanded usage of the available violet-excitable dyes including the SuperBright and Brilliant Violet dye series. The instrument uses acoustic focusing to center cells in the stream, allowing for significantly increased acquisition speeds (up to 1000uL per minute) with little to no loss in resolution. It is also equipped with an auto-sampler for regular and deep-well 96 and 384 well plates.
ThermoFisher Attune NxT V4 Flow Cytometer: “Lightning”
Location: WIMR 7018D
The Attune NxT is a benchtop analysis cytometer with 4 lasers (488, 405, 561, and 633nm). Capable of collecting up to 16 parameters: 2 scatter and 14 fluorescent, the optical bench is user-configurable to accommodate unusual fluorochromes and unique combinations. The instrument uses acoustic focusing to center cells in the stream, allowing for significantly increased acquisition speeds (up to 1000uL per minute) with little to no loss in resolution. It is also equipped with an auto-sampler for regular and deep-well 96 and 384 well plates.
ThermoFisher Attune NxT V6 Flow Cytometer: “River”
Location: Biotech 2360
The Attune NxT is a benchtop analysis cytometer with 4 lasers (488, 405, 561, and 633nm). Capable of collecting up to 16 parameters: 2 scatter and 14 fluorescent, the optical bench is user-configurable to accommodate unusual fluorochromes and unique combinations. The V6 model is configured with 6 channels off the violet laser, allowing expanded usage of the available violet-excitable dyes including the SuperBright and Brilliant Violet dye series.
The instrument uses acoustic focusing to center cells in the stream, allowing for significantly increased acquisition speeds (up to 1000uL per minute) with little to no loss in resolution. It is also equipped with an auto-sampler for regular and deep-well 96 and 384 well plates.
ThermoFisher Attune NxT Flow Cytometer: “Lava”
Location: CSC K4/523
Managed by the UWCCC Flow Lab and located in RASR, this instrument is reserved for the analysis of radiotherapy samples. Users must have completed the Flow Lab’s training program, and Radiation Safety Training in order to use this instrument.
The Attune NxT is a benchtop analysis cytometer with 4 lasers (488, 405, 561, and 633nm). Capable of collecting up to 16 parameters: 2 scatter and 14 fluorescent, the optical bench is user-configurable to accommodate unusual fluorochromes and unique combinations. The instrument uses acoustic focusing to center cells in the stream, allowing for significantly increased acquisition speeds (up to 1000uL per minute) with little to no loss in resolution. It is also equipped with an auto-sampler for regular and deep-well 96 and 384 well plates.
ThermoFisher Attune NxT V4 Flow Cutometer: “Peds”
Location: WIMR 4148A
Acquired through generous gifts from the Lion’s Club and the MACC Fund, this instrument is reserved for Pediatric Cancer Research Labs. Please Contact the Flow Lab for more information.
The Attune NxT is a benchtop analysis cytometer with 4 lasers (488, 405, 561, and 633nm). Capable of collecting up to 16 parameters: 2 scatter and 14 fluorescent, the optical bench is user-configurable to accommodate unusual fluorochromes and unique combinations. The instrument uses acoustic focusing to center cells in the stream, allowing for significantly increased acquisition speeds (up to 1000uL per minute) with little to no loss in resolution. It is also equipped with an auto-sampler for regular and deep-well 96 and 384 well plates.
BD LSR Fortessa
 Location: WIMR 7018D
Location: WIMR 7018D
The LSRII is a 5 laser (355nm, 405nm, 488nm, 561nm, and 640nm) 20 parameter (2 scatter and 18 fluorescence) benchtop analysis cytometer. The optical bench is user configurable for detection of less common fluorochromes and unique combinations. The Fortessa also has a High-Throughput Sampler for automated sampling of 96 and 384 well plates.
ImageStream
 Location: WIMR 7018A
Location: WIMR 7018A
The ImageStream MarkII is an imaging cytometer for cells in suspension. It has high resolution CCD cameras instead of photomultiplier tubes for detection and is equipped with 20x, 40x and 60x objectives, six detection channels and two lasers (488nm and 642nm).
BD FACSAria in BioBubble “Jayne” in Biotech 2360

Best Used For: Higher parameter sorts (6+ colors), sorts that require staff assistance, new users, infrequent sorts, unique sorts (nuclei, bacteria, yeast, cell cycle, and other unusual particles), sorts for downstream Single Cell Genomics, UV excitable dyes. BSL-1 and BSL-2 Only
If sorting yeast or bacteria, please reserve an additional 45 minutes at the end of your reservation for instrument cleaning.
All sort requests require a current Biological Safety Protocol number in which all materials brought for sorting are described and approved by the UW Office of Biological Safety for cell sorting in the Flow Lab.
Sort requests will not be approved without a current and relevant Biological Safety Protocol number.
Installed in March of 2019, this cell sorter is equipped with 5 lasers (488nm, 405nm, 633nm, 561nm, and 355nm) and 18 fluorescence detection channels. This instrument is contained in a BioBubble negative pressure containment unit and is strictly limited to BSL-2 or lower samples only. It is capable of sorting up to 4 populations simultaneously into tubes, single and multiple cell deposition into 6 – 384 well plates and other custom devices.
This Aria has been placed in the UW Biotechnology Center to support Single Cell Genomics pipelines as well as to provide cell sorting to central campus research labs. Located in the same building as the following cores; Gene Expression Center, DNA Sequencing, Genome Editing & Animal Models, and the Bioinformatics Resource Center, this instrument is uniquely positioned to provide highly purified cell subpopulations and single cell per well deposition into up to 384 well plates for downstream genomics assays.
Scheduling
The Flow Lab may adjust requested start times for sort appointments to optimize available instrument time.
Biosafety and Cell Sorting
Anything sorted in the Flow Lab MUST be described on your Lab’s Biological safety protocol. Cell sorting creates aerosols and additional biological safety precautions are required. The Flow Lab is able to sort samples of BSL-2 and below.
All sort requests require a current Biological Safety Protocol number in which all materials brought for sorting are described and approved by the UW Office of Biological Safety for cell sorting in the Flow Lab.
Sort requests will not be approved without a current and relevant Biological Safety Protocol number.
Flow Lab Hood Certification Dates
-
- Baker BDM401 BD FACSDiscover S8 “Sparky” Cell Sorter (7018B WIMR), SN 147503, Cert. Date: 11/18/25
Baker BCC300AMS Sony MA900 Cell Sorter (7018C WIMR), SN 138210, Cert. Date: 11/18/25
BioBubble Aria “Jayne” Cell Sorter (2360 Biotech), SN PU2525-0159, Cert. Date: 03/13/25
4′ Baker SterilGARD (7018E WIMR), SN 148774, Cert. Date: 11/19/25
6′ Baker SterilGARD (7018E WIMR), SN 150064, Cert. Date: 06/06/25
4′ Baker SterilGARD (2360 Biotech), SN 147996, Cert. Date: 03/13/25
- Baker BDM401 BD FACSDiscover S8 “Sparky” Cell Sorter (7018B WIMR), SN 147503, Cert. Date: 11/18/25
BD FACSAria with Biosafety Cabinet “Jill” in WIMR 7018B
RETIRED as of 04/11/25. Information remains posted for use in publications, posters, presentations. Please see Acknowledgements for info on adding relevant acknowledgements to your publication, poster or presentation.
 Best Used For: Higher parameter sorts (6+ colors), sorts that require staff assistance, new users, infrequent sorts, unique sorts (nuclei, bacteria, yeast, cell cycle, and other unusual particles), UV excitable dyes. BSL-2, Limited BSL2+, and NHP SPF
Best Used For: Higher parameter sorts (6+ colors), sorts that require staff assistance, new users, infrequent sorts, unique sorts (nuclei, bacteria, yeast, cell cycle, and other unusual particles), UV excitable dyes. BSL-2, Limited BSL2+, and NHP SPF
All sort requests require a current Biological Safety Protocol number in which all materials brought for sorting are described and approved by the UW Office of Biological Safety for cell sorting in the Flow Lab.
Sort requests will not be approved without a current and relevant Biological Safety Protocol number.
Cell sorting flow cytometer capable of sorting up to 4 populations simultaneously into tubes, single and multiple cell deposition into 6 – 384 well plates and other custom devices. Equipped with 5 lasers: 488nm, 405nm, 640nm, 532nm, and 355nm, 16 fluorescence detection channels, and ability to customize emission filters for specific fluorochromes. Contained in custom biological safety cabinet for sorting up to BSL-2 single cell suspensions.
The BD FACSAria “Jill” is an older model for which BD no longer offers service contracts. We have retired the instrument and are no longer accepting reservations.

 The BD FACSDiscover S8 Cell Sorter performs image-enabled cell sorting and spectral flow cytometry. Learn more about the instrument here.
The BD FACSDiscover S8 Cell Sorter performs image-enabled cell sorting and spectral flow cytometry. Learn more about the instrument here.
The instrument has 5 excitation lasers (349nm, 405nm, 488nm, 561nm, 637nm) for spectral detection across 78 channels. In addition, 3 imaging scatter parameters and up to 3 fluorescence channels can be defined using 488nm excitation.
Sorting Capabilities: up to 6 populations simultaneously, ability to perform single & multiple cell deposition into up to 384-well plates, microscope slides, and custom devices.
Information about using FlowJo software and the BD CellView(TM) plug-in to analyze data and images from the S8 can be found in this description and this video.
To learn more about how this instrument can advance your research email the Flow Lab at uwflow@uwcarbone.wisc.edu.
BD FACSDiscover S8 Instrument Map: https://cancer.wisc.edu/research/wp-content/uploads/2024/11/UWFlow-S8-Map-Spectral-Imaging.pdf
Biosafety and Cell Sorting
Anything sorted in the Flow Lab MUST be described on your Lab’s Biological safety protocol. Cell sorting creates aerosols and additional biological safety precautions are required. The Flow Lab is able to sort samples of BSL-2 and below.
All sort requests require a current Biological Safety Protocol number in which all materials brought for sorting are described and approved by the UW Office of Biological Safety for cell sorting in the Flow Lab.
Sort requests will not be approved without a current and relevant Biological Safety Protocol number.
Flow Lab Hood Certification Dates
-
- Baker BDM401 BD FACSDiscover S8 “Sparky” Cell Sorter (7018B WIMR), SN 147503, Cert. Date: 11/18/25
Baker BCC300AMS Sony MA900 Cell Sorter (7018C WIMR), SN 138210, Cert. Date: 11/18/25
BioBubble Aria “Jayne” Cell Sorter (2360 Biotech), SN PU2525-0159, Cert. Date: 03/13/25
4′ Baker SterilGARD (7018E WIMR), SN 148774, Cert. Date: 11/19/25
6′ Baker SterilGARD (7018E WIMR), SN 150064, Cert. Date: 06/06/25
4′ Baker SterilGARD (2360 Biotech), SN 147996, Cert. Date: 03/13/25
- Baker BDM401 BD FACSDiscover S8 “Sparky” Cell Sorter (7018B WIMR), SN 147503, Cert. Date: 11/18/25


The Sony MA900 Multi-Application Cell Sorter with sample and collection devices.
Best Used For: Fluorescent Proteins (GFP, mCherry, tdTomato, etc.), lower parameter sorts (1-8 colors), End-User Operation (self-sorting), After-hours and Weekend Self-Sorting. BSL-1 and BSL-2 Only.
All sort requests require a current Biological Safety Protocol number in which all materials brought for sorting are described and approved by the UW Office of Biological Safety for cell sorting in the Flow Lab.
Sort requests will not be approved without a current and relevant Biological Safety Protocol number.
Location: WIMR 7018C
The Sony MA900 is a highly automated cell sorter allowing for end-user self-operation, and we also provide training for researchers to operate this instrument independently. After training, researchers can schedule self-run appointments at any time, including after-hours and on weekends to accommodate unusual time points, long pre-processing workflows, and clinical research samples with later arrival times. We also offer staff-operated appointments on Mondays, Wednesdays, Thursdays, and Fridays.
Training is composed of 2 appointments:
- Start-up & QC Training – available in the mornings on Tuesdays, Wednesdays, and Thursdays
- Cell Sorting & Shutdown Training – available on Tuesdays, Wednesdays, and Thursdays. Customers should bring stained cells for sorting. Training will include setting up the sort, adjusting sensitivity, compensation, sorting, saving a template, rigor and reproducibility considerations, and instrument shutdown.
- Staff is available for additional training/assistance as needed. Please email the Flow Lab at uwflow@uwcarbone.wisc.edu if you need staff assistance during a self-run reservation during set-up, or for a new assay.
The MA900 is capable of sorting up to 4 populations simultaneously into tubes, single and multiple cell deposition into 6 – 384 well plates and other custom devices, including PCR plates. Equipped with 4 lasers arranged in co-linear pairs: 488nm & 561nm, and, 405nm and 634nm, 12 fluorescence detection channels, and ability to customize emission filters for specific fluorochromes. Contained in custom biological safety cabinet for sorting up to BSL-2 single cell suspensions.
Sony MA900 Fluorochrome and Filter Guide
Sony MA900 Manufacturer Webpage
Biosafety and Cell Sorting
Anything sorted in the Flow Lab MUST be described on your Lab’s Biological safety protocol. Cell sorting creates aerosols and additional biological safety precautions are required. The Flow Lab is able to sort samples of BSL-2 and below.
All sort requests require a current Biological Safety Protocol number in which all materials brought for sorting are described and approved by the UW Office of Biological Safety for cell sorting in the Flow Lab.
Sort requests will not be approved without a current and relevant Biological Safety Protocol number.
Flow Lab Hood Certification Dates
-
- Baker BDM401 BD FACSDiscover S8 “Sparky” Cell Sorter (7018B WIMR), SN 147503, Cert. Date: 11/18/25
Baker BCC300AMS Sony MA900 Cell Sorter (7018C WIMR), SN 138210, Cert. Date: 11/18/25
BioBubble Aria “Jayne” Cell Sorter (2360 Biotech), SN PU2525-0159, Cert. Date: 03/13/25
4′ Baker SterilGARD (7018E WIMR), SN 148774, Cert. Date: 11/19/25
6′ Baker SterilGARD (7018E WIMR), SN 150064, Cert. Date: 06/06/25
4′ Baker SterilGARD (2360 Biotech), SN 147996, Cert. Date: 03/13/25
- Baker BDM401 BD FACSDiscover S8 “Sparky” Cell Sorter (7018B WIMR), SN 147503, Cert. Date: 11/18/25
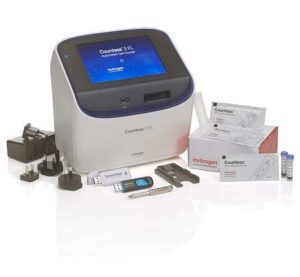
Location: WIMR 7016
The Countess 3 FL Automated Cell Counter is a benchtop assay platform equipped with state-of-the-art optics, fully automated focus and lighting capability, and image analysis software driven by a neural network-based machine-learning algorithm, for rapid assessment of primary and immortalized cell lines. The Countess 3 FL counter is capable of brightfield or fluorescence illumination with three-channel flexibility (brightfield and two optional fluorescence channels) enabling researchers to count cells, monitor fluorescent protein expression, gain insights to apoptotic processes, and measure cell viability.
• Accurate—eliminates the subjectivity of manual cell counting and user-to-user variability
• Flexible—accepts up to two EVOS light cubes, user selectable from fifteen fluorescent channels
• Fast—counts live and dead cells, measures viability, reports average cell size, and saves data with a single touch, in about 20 seconds
• Convenient—requires no cleaning or routine maintenance; high-resolution capacitive touchscreen and simple user interface provide quick startup and requires minimal training
Filter cubes available in Flow Lab: GFP, PI/RFP/mCherry, DAPI
Counting Chambers available at cost from Flow Lab or order from Shop@UW: Cat# C10228 (Box of 50), Cat# C10312 (Box of 500)
Picture and description from ThermoFisher website, more info here: Countess 3FL Automated Cell Counter
 Location: WIMR 7016
Location: WIMR 7016
The Nexcelom Cellometer Auto T4 Cell Viability Counter is a PC-based instrument for one-step 4x bright field image capture and analysis. This state-of-the-art cell counter performs count, size, and Trypan Blue viability analyses that are 10x faster and more accurate than manual counting. This instrument is ideal for cell lines and purified primary cells, including MCF-7 breast cancer cells, PC3 prostate cancer cells, and 1600 other cell types, due to its advanced software that assesses clumpy cells and omits debris.
This is a slide based cell counter and requires Nexcelom counting slides. There are 2 tests per slide and the slides can be purchased individually or in packs of 75 from the Flow Lab, or from a variety of ShopUW+ vendors.
Offline data analysis computers are available to laboratory users only and are located at the Clinical Science Center in room K4/532, and at the Biotech Center in room 2360. Computers are loaded with a variety of analysis packages including FlowJo, FCS Express, SpectroFlo, ModFit, WinList, and IDEAS ImageStream analysis software, Microsoft Office, and Adobe Illustrator.
There is no charge for the use of these computers and are for the exclusive use for the analysis of data generated at this laboratory. Computers are available on a first-come, first-served walkup basis for users of the facility. Contact Flow Lab staff for access to these rooms.
Pricing
Hourly Rates – Effective January 1, 2025 |
|||||
Service |
UWCCC Member |
Non-Member (UW) |
Wm S Middleton VA |
External Academic |
Industry |
| Cytometer Training / Assisted Staff Run
Attune, Aurora, LSR Fortessa, ImageStream |
$117.82 | $157.10 | $174.38 | $251.35 | $322.04 |
| Cytometer Self Run
Attune, Aurora, LSR Fortessa, ImageStream |
$70.98 | $94.64 | $105.05 | $151.42 | $194.00 |
| Staff Assistance (Cytometers)* | $46.84 | $62.46 | $69.33 | $99.94 | $128.04 |
| LSR Fortessa Weekend Use Training | $117.82 | $157.10 | $174.38 | $251.35 | $322.04 |
| Staff Assisted De-Clog Fee* | $131.25 | $175.00 | $194.25 | $280.00 | $358.75 |
| Cell Sorting Training /Assisted Staff Run
Aria, Sony MA900, BD S8 |
$134.14 | $178.86 | $198.53 | $286.17 | $366.66 |
| Sorter Set-up Fee (Staff Run) | $67.07 | $89.43 | $99.27 | $143.09 | $183.33 |
| Cell Sorting Self Run
Aria, Sony MA900, BD S8 |
$95.82 | $127.76 | $141.81 | $204.41** | $261.90** |
| Staff Assistance (Cell Sorters)* | $38.33 | $51.10 | $56.72 | $81.76 | $104.76 |
| Consultation (Discussion Only) | No Charge | $33.12 | $36.76 | $52.99 | $67.89 |
| Sort Consultation (Req’d before 1st Sort) | No Charge | No Charge | No Charge | $52.99 | $67.89 |
| Assay Services | $131.25 | $175.00 | $194.25 | $280.00 | $358.75 |
Assay Services include: Protocol Optimization & Development, Panel Design, Data Analysis, Data Reporting & Figure Presentation. |
|||||
*15 minute minimum charge, staff assistance add on for additional staff assistance with self-run reservations**after-hours and weekend usage unavailable to off-campus users Aria Self-Use Training evaluated on a case-by-case basis. Contact Flow Lab for more info. |
|||||
Per Usage Fees |
|||||
Service |
UWCCC Member |
Non-Member |
Wm S Middleton VA |
External Academic |
Off Campus |
| Lecture Series (Req’d before using instrumentation) | $83.57 | $115.98 | $128.73 | $183.96 | $276.02 |
| Countess & Cellometer Cell Counters | Customer purchases counting slides | ||||

