Kari Wisinski, MD – Genetic and Epigenetic Mechanisms Program
NCI9782 Talazoparib
A Phase 1 study (UM1CA186716; NCT02317874) on Talazoparib in combination with Carboplatin and Paclitaxel that reported myelosuppression, but durable clinical activity, at relatively low doses and an intermittent schedule. Talazoparib plasma concentrations were quantified by the CPL Analytical Laboratory using a validated LC/MS-MS assay. Peripheral blood mononuclear cells (PBMC) were collected and processed by CPL for quantitative analysis:
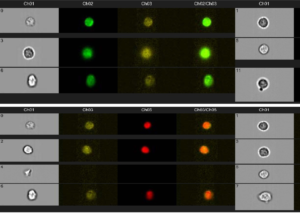
Shown here is an analysis of rad51 foci, and MFI for γH2AX and PAR levels. Drug-treated cells (or untreated controls) were isolated from patient PBMCs and stained with antibodies to rad51, γH2AX, and PAR, along with a nuclear dye (DRAQ5). After staining, the cells were sorted by FlowImage in the Flow Lab, to determine MFI and to count individual foci.
Leal, et al. A phase I study of talazoparib (BMN 673) combined with carboplatin and paclitaxel in patients with advanced solid tumors (NCI9782). Cancer Med 2022
David Jarrard, MD – Genetic and Epigenetic Mechanisms Program
DDC supported a phase 2 study lead by Jarrard (GEM)
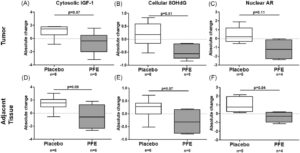
This was the first randomized-controlled study of pomegranate fruit extract (PFE) over an extended period in an actively surveilled population.
PFE showed promising tissue-level changes in oxidative stress and androgen receptor expression.
CPL enabled this research through quantification of biomarkers, metabolites, and drug levels in serum, plasma, and urine
Jarrard D, Filon M, Huang W, et al. A phase II randomized placebo-controlled trial of pomegranate fruit extract in men with localized prostate cancer undergoing active surveillance. The Prostate. 2021; 81:41-49.
Howard Bailey, MD – UWCCC Director, and CPC Program Member
The Cancer Pharmacology Lab developed and validated an LC/MS/MS analytical assay for a first in human clinical trial of UAB30. UAB30 is a novel retinoid being developed as a chemoprevention agent and a therapeutic agent for breast cancer. The primary endpoint of this trial was to evaluate the single dose PK in normal volunteers, with the primary hypothesis that UAB30 would be orally available. This study demonstrated oral availability, dose proportionality as depicted below, primarily hepatic metabolism and a half-life of approximately eight hours for UAB30.
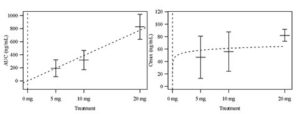
Means and Standard Error Bars of AUC and Cmax by UAB30 treatment group shown, along with fitted curves representing chosen models.
Glenn Liu, MD – Developmental Therapeutics Program
The Cancer Pharmacology Lab developed and validated an LC/MS/MS (liquid chromatography tandem mass spectrometry) assay for the analysis of sunitinib and performed an ELISA assay for VEGF, which were utilized in NCI #7898, “Pharmacodynamic Study Using FLT PET/CT in Patients with Renal Cell Cancer and Other Solid Malignancies Treated with Sunitinib Malate.”
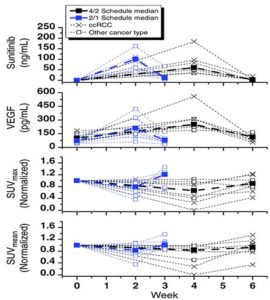
Multivariate analysis demonstrated that plasma VEGF levels independently predict tumor proliferation during sunitinib treatment. PK and PD parameters at time points throughout the sunitinib cycle are shown. Thin dotted lines show individual patient results. (ccRCC, clear cell renal cell carcinoma.)
More about Cancer Pharmacology
Selected Publications
Funding received from the UWCCC Cancer Center Support Grant (CCSG) for published research, including the use of UWCCC Shared Resources to generate or analyze data, or conduct clinical trials, needs to reference the UWCCC. See Acknowledging UWCCC in Publications, Posters, and Presentations.
View the UW Drug Development Core (DDC) page for more information on other UW–Madison laboratory facilities that provide state-of-the-art instrumentation and expert scientific staff.
| Analytical |
| Scribano CM, Wan J, Esbona K, Tucker JB, Lasek A, Zhou AS, Zasadil LM, Molini R, Fitzgerald J, Lager AM, Laffin JJ, Correia-Staudt K, Wisinski KB, Tevaarwerk AJ, O’Regan R, McGregor SM, Fowler AM, Chappell RJ, Bugni TS, Burkard ME, Weaver BA. Chromosomal instability sensitizes patient breast tumors to multipolar divisions induced by paclitaxel. Sci Transl Med. 2021 SEP 08; 13(610):eabd4811. DOI: 10.1126/scitranslmed.abd4811. 2021 Sep 8. PubMed PMID: 34516829; PMCID: PMC8612166. |
| Nehra G, Andrews S, Rettig J, Gould MN, Haag JD, Howard SP, Thorne RG. Intranasal administration of the chemotherapeutic perillyl alcohol results in selective delivery to the cerebrospinal fluid in rats. Sci Rep. 2021 MAR 18; 11(1):6351. DOI: 10.1038/s41598-021-85293-4. 2021 Mar 18. PubMed PMID: 33737566; PMCID: PMC7973779. |
| Jarrard D, Filon M, Huang W, Havighurst T, DeShong K, Kim K, Konety BR, Saltzstein D, Mukhtar H, Wollmer B, Suen C, House MG, Parnes HL, Bailey HH. A phase II randomized placebo-controlled trial of pomegranate fruit extract in men with localized prostate cancer undergoing active surveillance. Prostate. 2021 JAN 01; 81(1):41-49. DOI: 10.1002/pros.24076. 2020 Oct 23. PubMed PMID: 33095939. |
| Murphy AG, Zahurak M, Shah M, Weekes CD, Hansen A, Siu LL, Spreafico A, LoConte N, Anders NM, Miles T, Rudek MA, Doyle LA, Nelkin B, Maitra A, Azad NS. A Phase I Study of Dinaciclib in Combination With MK-2206 in Patients With Advanced Pancreatic Cancer. Clin Transl Sci. 2020 NOV 01; 13(6):1178-1188. DOI: 10.1111/cts.12802. 2020 Aug 1. PubMed PMID: 32738099; PMCID: PMC7719383. |
| Kolesar JM, Andrews S, Green H, Havighurst TC, Wollmer BW, DeShong K, Laux DE, Krontiras H, Muccio DD, Kim K, Grubbs CJ, House MG, Parnes HL, Heckman-Stoddard BM, Bailey HH. A Randomized, Placebo-Controlled, Double-Blind, Dose Escalation, Single Dose, and Steady-State Pharmacokinetic Study of 9cUAB30 in Healthy Volunteers. Cancer Prev Res (Phila). 2019 DEC 01; 12(12):903-912. DOI: 10.1158/1940-6207.CAPR-19-0310. 2019 Sep 4. PubMed PMID: 31484659; PMCID: PMC7008944. |
| Lubner S, Feng Y, Mulcahy M, O’Dwyer P, Giang GY, Hinshaw JL, Deming D, Klein L, Teitelbaum U, Payne J, Engstrom P, Stella P, Meropol N, Benson A. E4206: AMG 706 and Octreotide in Patients with Low-Grade Neuroendocrine Tumors. Oncologist. 2018 SEP 01; 23(9):1006-e104. DOI: 10.1634/theoncologist.2018-0294. 2018 May 31. PubMed PMID: 29853660; PMCID: PMC6192662. |
| Scarpelli M, Rampurwala M, Eickhoff J, Carmichael L, Heideman J, Binger K, Kolesar J, Perlman S, Harrow K, Dukart G, Liang C, Jeraj R, Liu G, Bruce JY. Pharmacodynamic study using FLT PET/CT in advanced solid malignancies treated with a sequential combination of X-82 and docetaxel. Cancer Chemother. Pharmacol.. 2018 AUG 01; 82(2):211-219. DOI: 10.1007/s00280-018-3599-3. 2018 May 25. PubMed PMID: 29802443; PMCID: PMC7205037. |
| Kyriakopoulos CE, Braden AM, Kolesar JM, Eickhoff JC, Bailey HH, Heideman J, Liu G, Wisinski KB. A phase I study of tivantinib in combination with temsirolimus in patients with advanced solid tumors. Invest New Drugs. 2017 JUN 01; 35(3):290-297. DOI: 10.1007/s10637-016-0418-8. 2016 Dec 21. PubMed PMID: 28004284; PMCID: PMC5809175. |
| Gee JR, Saltzstein DR, Kim K, Kolesar J, Huang W, Havighurst TC, Wollmer BW, Stublaski J, Downs T, Mukhtar H, House MG, Parnes HL, Bailey HH. A Phase II Randomized, Double-blind, Presurgical Trial of Polyphenon E in Bladder Cancer Patients to Evaluate Pharmacodynamics and Bladder Tissue Biomarkers. Cancer Prev Res (Phila). 2017 MAY 01; 10(5):298-307. DOI: 10.1158/1940-6207.CAPR-16-0167. 2017 Mar 21. PubMed PMID: 28325826; PMCID: PMC5503683. |
| Rampurwala M, Wisinski KB, Burkard ME, Ehsani S, O’Regan RM, Carmichael L, Kim K, Kolesar J, Tevaarwerk AJ. Phase 1b study of orteronel in postmenopausal women with hormone-receptor positive (HR+) metastatic breast cancer. Invest New Drugs. 2017 FEB 01; 35(1):87-94. DOI: 10.1007/s10637-016-0403-2. 2016 Nov 8. PubMed PMID: 27826831; PMCID: PMC5590750. |
| SAS |
| Gregory GP, Kumar SK, Wang D, Mahadevan D, Walker PA, Wagner-Johnston ND, Escobar C, Bannerji R, Bhutani D, Chang JE, Hernandez-Ilizaliturri FJ, Klein A, Pagel JM, Rybka W, Yee AJ, Mohrbacher A, Huang M, Farooqui MZH, Marinello P, Quach H. Pembrolizumab plus dinaciclib in patients with hematologic malignancies: the phase 1b KEYNOTE-155 study. Blood Adv. 2021 DEC 31; . DOI: 10.1182/bloodadvances.2021005872. 2021 Dec 31. PubMed PMID: 34972202. |
| Costa LJ, Chhabra S, Medvedova E, Dholaria BR, Schmidt TM, Godby KN, Silbermann R, Dhakal B, Bal S, Giri S, D’Souza A, Hall A, Hardwick P, Omel J, Cornell RF, Hari P, Callander NS. Daratumumab, Carfilzomib, Lenalidomide, and Dexamethasone With Minimal Residual Disease Response-Adapted Therapy in Newly Diagnosed Multiple Myeloma. J. Clin. Oncol.. 2021 DEC 13; :JCO2101935. DOI: 10.1200/JCO.21.01935. 2021 Dec 13. PubMed PMID: 34898239. |
| Liu J, Burris H, Wang JS, Barroilhet L, Gutierrez M, Wang Y, Vaze A, Commerford R, Royer-Joo S, Choeurng V, Humke E, Moore K. An open-label phase I dose-escalation study of the safety and pharmacokinetics of DMUC4064A in patients with platinum-resistant ovarian cancer. Gynecol. Oncol.. 2021 DEC 01; 163(3):473-480. DOI: 10.1016/j.ygyno.2021.09.023. 2021 Oct 6. PubMed PMID: 34627611. |
| Emamekhoo H, Olsen MR, Carthon BC, Drakaki A, Percent IJ, Molina AM, Cho DC, Bendell JC, Gordan LN, Rezazadeh Kalebasty A, George DJ, Hutson TE, Arrowsmith ER, Zhang J, Zoco J, Johansen JL, Leung DK, Tykodi SS. Safety and efficacy of nivolumab plus ipilimumab in patients with advanced renal cell carcinoma with brain metastases: CheckMate 920. Cancer. 2021 NOV 16; . DOI: 10.1002/cncr.34016. 2021 Nov 16. PubMed PMID: 34784056. |
| Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, Abdallah AO, Callander N, Sborov D, Suvannasankha A, Weisel K, Voorhees PM, Womersley L, Baron J, Piontek T, Lewis E, Opalinska J, Gupta I, Cohen AD. Longer term outcomes with single-agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma: 13-month follow-up from the pivotal DREAMM-2 study. Cancer. 2021 NOV 15; 127(22):4198-4212. DOI: 10.1002/cncr.33809. 2021 Jul 27. PubMed PMID: 34314018; PMCID: PMC8597112. |
| Connolly RM, Zhao F, Miller KD, Lee MJ, Piekarz RL, Smith KL, Brown-Glaberman UA, Winn JS, Faller BA, Onitilo AA, Burkard ME, Budd GT, Levine EG, Royce ME, Kaufman PA, Thomas A, Trepel JB, Wolff AC, Sparano JA. E2112: Randomized Phase III Trial of Endocrine Therapy Plus Entinostat or Placebo in Hormone Receptor-Positive Advanced Breast Cancer. A Trial of the ECOG-ACRIN Cancer Research Group. J. Clin. Oncol.. 2021 OCT 01; 39(28):3171-3181. DOI: 10.1200/JCO.21.00944. 2021 Aug 6. PubMed PMID: 34357781; PMCID: PMC8478386. |
| Pal SK, Frankel PH, Mortazavi A, Milowsky M, Vaishampayan U, Parikh M, Lyou Y, Weng P, Parikh R, Teply B, Dreicer R, Emamekhoo H, Michaelson D, Hoimes C, Zhang T, Srinivas S, Kim WY, Cui Y, Newman E, Lara PN. Effect of Cisplatin and Gemcitabine With or Without Berzosertib in Patients With Advanced Urothelial Carcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021 OCT 01; 7(10):1536-1543. DOI: 10.1001/jamaoncol.2021.3441. PubMed PMID: 34436521; PMCID: PMC8391778. |
| Gopal AK, Popat R, Mattison RJ, Menne T, Bloor A, Gaymes T, Khwaja A, Juckett M, Chen Y, Cotter MJ, Mufti GJ. A Phase I trial of talazoparib in patients with advanced hematologic malignancies. Int J Hematol Oncol. 2021 SEP 01; 10(3):IJH35. DOI: 10.2217/ijh-2021-0004. 2021 Oct 22. PubMed PMID: 34840720; PMCID: PMC8609999. |
| Malhotra J, Nikolinakos P, Leal T, Lehman J, Morgensztern D, Patel JD, Wrangle JM, Curigliano G, Greillier L, Johnson ML, Ready N, Robinet G, Lally S, Maag D, Valenzuela R, Blot V, Besse B. A Phase 1-2 Study of Rovalpituzumab Tesirine in Combination With Nivolumab Plus or Minus Ipilimumab in Patients With Previously Treated Extensive-Stage SCLC. J Thorac Oncol. 2021 SEP 01; 16(9):1559-1569. DOI: 10.1016/j.jtho.2021.02.022. 2021 Feb 27. PubMed PMID: 33652156. |
| Mayer IA, Zhao F, Arteaga CL, Symmans WF, Park BH, Burnette BL, Tevaarwerk AJ, Garcia SF, Smith KL, Makower DF, Block M, Morley KA, Jani CR, Mescher C, Dewani SJ, Tawfik B, Flaum LE, Mayer EL, Sikov WM, Rodler ET, Wagner LI, DeMichele AM, Sparano JA, Wolff AC, Miller KD. Randomized Phase III Postoperative Trial of Platinum-Based Chemotherapy Versus Capecitabine in Patients With Residual Triple-Negative Breast Cancer Following Neoadjuvant Chemotherapy: ECOG-ACRIN EA1131. J. Clin. Oncol.. 2021 AUG 10; 39(23):2539-2551. DOI: 10.1200/JCO.21.00976. 2021 Jun 6. PubMed PMID: 34092112; PMCID: PMC8577688. |
| Tagawa ST, Balar AV, Petrylak DP, Kalebasty AR, Loriot Y, Fléchon A, Jain RK, Agarwal N, Bupathi M, Barthelemy P, Beuzeboc P, Palmbos P, Kyriakopoulos CE, Pouessel D, Sternberg CN, Hong Q, Goswami T, Itri LM, Grivas P. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol.. 2021 AUG 01; 39(22):2474-2485. DOI: 10.1200/JCO.20.03489. 2021 Apr 30. PubMed PMID: 33929895; PMCID: PMC8315301. |
| Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leal TA, Riess JW, Jensen E, Zhao B, Pietanza MC, Brahmer JR. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50. J. Clin. Oncol.. 2021 JUL 20; 39(21):2339-2349. DOI: 10.1200/JCO.21.00174. 2021 Apr 19. PubMed PMID: 33872070; PMCID: PMC8280089. |
| Boyer M, Şendur MAN, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, Yumuk PF, Orlandi FJ, Leal TA, Molinier O, Soparattanapaisarn N, Langleben A, Califano R, Medgyasszay B, Hsia TC, Otterson GA, Xu L, Piperdi B, Samkari A, Reck M. Pembrolizumab Plus Ipilimumab or Placebo for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50%: Randomized, Double-Blind Phase III KEYNOTE-598 Study. J. Clin. Oncol.. 2021 JUL 20; 39(21):2327-2338. DOI: 10.1200/JCO.20.03579. 2021 Jan 29. PubMed PMID: 33513313. |
| Drake CG, Pachynski RK, Subudhi SK, McNeel DG, Antonarakis ES, Bauer TM, Lauer P, Brockstedt D, Patricia D, Wade M, Zudaire E, Bandyopadhyay N, Parasrampuria DA, Girgis S, Mason GE, Knoblauch RE, Stone N, Infante JR, Gottardis MM, Fong L. Safety and preliminary immunogenicity of JNJ-64041809, a live-attenuated, double-deleted Listeria monocytogenes-based immunotherapy, in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis.. 2021 JUL 13; . DOI: 10.1038/s41391-021-00402-8. 2021 Jul 13. PubMed PMID: 34257408. |
| Wefel JS, Armstrong TS, Pugh SL, Gilbert MR, Wendland MM, Brachman DG, Roof KS, Brown PD, Crocker IR, Robins HI, Hunter G, Won M, Mehta MP. Neurocognitive, symptom, and health-related quality of life outcomes of a randomized trial of bevacizumab for newly diagnosed glioblastoma (NRG/RTOG 0825). Neuro-oncology. 2021 JUL 01; 23(7):1125-1138. DOI: 10.1093/neuonc/noab011. PubMed PMID: 33515019; PMCID: PMC8661434. |
| Naing A, Thistlethwaite F, De Vries EGE, Eskens FALM, Uboha N, Ott PA, LoRusso P, Garcia-Corbacho J, Boni V, Bendell J, Autio KA, Randhawa M, Durm G, Gil-Martin M, Stroh M, Hannah AL, Arkenau HT, Spira A. CX-072 (pacmilimab), a Probody ® PD-L1 inhibitor, in advanced or recurrent solid tumors (PROCLAIM-CX-072): an open-label dose-finding and first-in-human study. J Immunother Cancer. 2021 JUL 01; 9(7). DOI: 10.1136/jitc-2021-002447. PubMed PMID: 34301809; PMCID: PMC8311335. |
| Tolaney SM, Kalinsky K, Kaklamani VG, D’Adamo DR, Aktan G, Tsai ML, O’Regan RM, Kaufman PA, Wilks ST, Andreopoulou E, Patt DA, Yuan Y, Wang G, Savulsky C, Xing D, Kleynerman E, Karantza V, Diab S. Eribulin Plus Pembrolizumab in Patients with Metastatic Triple-Negative Breast Cancer (ENHANCE 1): A Phase Ib/II Study. Clin. Cancer Res.. 2021 JUN 01; 27(11):3061-3068. DOI: 10.1158/1078-0432.CCR-20-4726. 2021 Mar 16. PubMed PMID: 33727258. |
| Pullarkat VA, Lacayo NJ, Jabbour E, Rubnitz JE, Bajel A, Laetsch TW, Leonard J, Colace SI, Khaw SL, Fleming SA, Mattison RJ, Norris R, Opferman JT, Roberts KG, Zhao Y, Qu C, Badawi M, Schmidt M, Tong B, Pesko JC, Sun Y, Ross JA, Vishwamitra D, Rosenwinkel L, Kim SY, et al. Venetoclax and Navitoclax in Combination with Chemotherapy in Patients with Relapsed or Refractory Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma. Cancer Discov. 2021 JUN 01; 11(6):1440-1453. DOI: 10.1158/2159-8290.CD-20-1465. 2021 Feb 16. PubMed PMID: 33593877. |
| Nooka AK, Weisel K, van de Donk NW, Routledge D, Otero PR, Song K, Quach H, Callander N, Minnema MC, Trudel S, Jackson NA, Ahlers CM, Im E, Cheng S, Smith L, Hareth N, Ferron-Brady G, Brouch M, Montes de Oca R, Paul S, Holkova B, Gupta I, Kremer BE, Richardson P. Belantamab mafodotin in combination with novel agents in relapsed/refractory multiple myeloma: DREAMM-5 study design. Future Oncol. 2021 JUN 01; 17(16):1987-2003. DOI: 10.2217/fon-2020-1269. 2021 Mar 8. PubMed PMID: 33682447. |
| Moschos SJ, Eroglu Z, Khushalani NI, Kendra KL, Ansstas G, In GK, Wang P, Liu G, Collichio FA, Googe PB, Carson CC, McKinnon K, Wang HH, Nikolaishvilli-Feinberg N, Ivanova A, Arrowood CC, Garrett-Mead N, Conway KC, Edmiston SN, Ollila DW, Serody JS, Thomas NE, Ivy SP, Agrawal L, Dees EC. Targeting the IL-2 inducible kinase in melanoma; a phase 2 study of ibrutinib in systemic treatment-refractory distant metastatic cutaneous melanoma: preclinical rationale, biology, and clinical activity (NCI9922). Melanoma Res.. 2021 APR 01; 31(2):162-172. DOI: 10.1097/CMR.0000000000000726. PubMed PMID: 33661190; PMCID: PMC8025369. |
| Peereboom DM, Ye X, Mikkelsen T, Lesser GJ, Lieberman FS, Robins HI, Ahluwalia MS, Sloan AE, Grossman SA. A Phase II and Pharmacodynamic Trial of RO4929097 for Patients With Recurrent/Progressive Glioblastoma. Neurosurgery. 2021 JAN 13; 88(2):246-251. DOI: 10.1093/neuros/nyaa412. PubMed PMID: 33027815; PMCID: PMC7919338. |
| Usmani SZ, Hoering A, Ailawadhi S, Sexton R, Lipe B, Hita SF, Valent J, Rosenzweig M, Zonder JA, Dhodapkar M, Callander N, Zimmerman T, Voorhees PM, Durie B, Rajkumar SV, Richardson PG, Orlowski RZ. Bortezomib, lenalidomide, and dexamethasone with or without elotuzumab in patients with untreated, high-risk multiple myeloma (SWOG-1211): primary analysis of a randomised, phase 2 trial. Lancet Haematol. 2021 JAN 01; 8(1):e45-e54. DOI: 10.1016/S2352-3026(20)30354-9. 2020 Dec 22. PubMed PMID: 33357482; PMCID: PMC8601389. |
| McKay RR, McGregor BA, Xie W, Braun DA, Wei X, Kyriakopoulos CE, Zakharia Y, Maughan BL, Rose TL, Stadler WM, McDermott DF, Harshman LC, Choueiri TK. Optimized Management of Nivolumab and Ipilimumab in Advanced Renal Cell Carcinoma: A Response-Based Phase II Study (OMNIVORE). J. Clin. Oncol.. 2020 DEC 20; 38(36):4240-4248. DOI: 10.1200/JCO.20.02295. 2020 Oct 27. PubMed PMID: 33108238; PMCID: PMC7768333. |
| Kumar SK, Jacobus SJ, Cohen AD, Weiss M, Callander N, Singh AK, Parker TL, Menter A, Yang X, Parsons B, Kumar P, Kapoor P, Rosenberg A, Zonder JA, Faber E, Lonial S, Anderson KC, Richardson PG, Orlowski RZ, Wagner LI, Rajkumar SV. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol.. 2020 OCT 01; 21(10):1317-1330. DOI: 10.1016/S1470-2045(20)30452-6. 2020 Aug 28. PubMed PMID: 32866432; PMCID: PMC7591827. |
| Dudek AZ, Liu LC, Fischer JH, Wiley EL, Sachdev JC, Bleeker J, Hurley RW, Tonetti DA, Thatcher GRJ, Venuti RP, O’Regan RM. Phase 1 study of TTC-352 in patients with metastatic breast cancer progressing on endocrine and CDK4/6 inhibitor therapy. Breast Cancer Res. Treat.. 2020 OCT 01; 183(3):617-627. DOI: 10.1007/s10549-020-05787-z. 2020 Jul 22. PubMed PMID: 32696319. |
| Bible KC, Menefee ME, Lin CJ, Millward MJ, Maples WJ, Goh BC, Karlin NJ, Kane MA, Adkins DR, Molina JR, Donehower RC, Lim WT, Flynn PJ, Richardson RL, Traynor AM, Rubin J, LoRusso PM, Smallridge RC, Burton JK, Suman VJ, Kumar A, Voss JS, Rumilla KM, Kipp BR, Chintakuntlawar AV, et al. An International Phase 2 Study of Pazopanib in Progressive and Metastatic Thyroglobulin Antibody Negative Radioactive Iodine Refractory Differentiated Thyroid Cancer. Thyroid. 2020 SEP 01; 30(9):1254-1262. DOI: 10.1089/thy.2019.0269. 2020 Jul 29. PubMed PMID: 32538690; PMCID: PMC7482116. |
| Hirbe AC, Eulo V, Moon CI, Luo J, Myles S, Seetharam M, Toeniskoetter J, Kershner T, Haarberg S, Agulnik M, Monga V, Milhem M, Parkes A, Robinson S, Okuno S, Attia S, Van Tine BA. A phase II study of pazopanib as front-line therapy in patients with non-resectable or metastatic soft-tissue sarcomas who are not candidates for chemotherapy. Eur. J. Cancer. 2020 SEP 01; 137:1-9. DOI: 10.1016/j.ejca.2020.06.016. 2020 Jul 23. PubMed PMID: 32712457. |
| Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, Hurvitz S, Loi S, Okines A, Abramson V, Bedard PL, Oliveira M, Mueller V, Zelnak A, DiGiovanna MP, Bachelot T, Chien AJ, O’Regan R, Wardley A, Conlin A, Cameron D, Carey L, Curigliano G, Gelmon K, Loibl S, et al. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J. Clin. Oncol.. 2020 AUG 10; 38(23):2610-2619. DOI: 10.1200/JCO.20.00775. 2020 May 29. PubMed PMID: 32468955; PMCID: PMC7403000. |
| Lee EQ, Zhang P, Wen PY, Gerstner ER, Reardon DA, Aldape KD, deGroot JF, Pan E, Raizer JJ, Kim LJ, Chmura SJ, Robins HI, Connelly JM, Battiste JD, Villano JL, Wagle N, Merrell RT, Wendland MM, Mehta MP. NRG/RTOG 1122: A phase 2, double-blinded, placebo-controlled study of bevacizumab with and without trebananib in patients with recurrent glioblastoma or gliosarcoma. Cancer. 2020 JUN 15; 126(12):2821-2828. DOI: 10.1002/cncr.32811. 2020 Mar 10. PubMed PMID: 32154928; PMCID: PMC7245544. |
| Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ, Bridgewater J, Harris WP, Murphy AG, Oh DY, Whisenant J, Lowery MA, Goyal L, Shroff RT, El-Khoueiry AB, Fan B, Wu B, Chamberlain CX, Jiang L, Gliser C, Pandya SS, Valle JW. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol.. 2020 JUN 01; 21(6):796-807. DOI: 10.1016/S1470-2045(20)30157-1. 2020 May 13. PubMed PMID: 32416072; PMCID: PMC7523268. |
| Dudek AZ, Liu LC, Gupta S, Logan TF, Singer EA, Joshi M, Zakharia YN, Lang JM, Schwarz JK, Al-Janadi A, Alva AS. Phase Ib/II Clinical Trial of Pembrolizumab With Bevacizumab for Metastatic Renal Cell Carcinoma: BTCRC-GU14-003. J. Clin. Oncol.. 2020 APR 10; 38(11):1138-1145. DOI: 10.1200/JCO.19.02394. 2020 Feb 25. PubMed PMID: 32097091; PMCID: PMC7145584. |
| Tarhini AA, Lee SJ, Hodi FS, Rao UNM, Cohen GI, Hamid O, Hutchins LF, Sosman JA, Kluger HM, Eroglu Z, Koon HB, Lawrence DP, Kendra KL, Minor DR, Lee CB, Albertini MR, Flaherty LE, Petrella TM, Streicher H, Sondak VK, Kirkwood JM. Phase III Study of Adjuvant Ipilimumab (3 or 10 mg/kg) Versus High-Dose Interferon Alfa-2b for Resected High-Risk Melanoma: North American Intergroup E1609. J. Clin. Oncol.. 2020 FEB 20; 38(6):567-575. DOI: 10.1200/JCO.19.01381. 2019 Dec 27. PubMed PMID: 31880964; PMCID: PMC7030886. |
| Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, Abdallah AO, Callander N, Lendvai N, Sborov D, Suvannasankha A, Weisel K, Karlin L, Libby E, Arnulf B, Facon T, Hulin C, Kortüm KM, Rodríguez-Otero P, Usmani SZ, Hari P, Baz R, Quach H, Moreau P, Voorhees PM, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol.. 2020 FEB 01; 21(2):207-221. DOI: 10.1016/S1470-2045(19)30788-0. 2019 Dec 16. PubMed PMID: 31859245. |
| Chen H, Kuhn J, Lamborn KR, Abrey LE, DeAngelis LM, Lieberman F, Robins HI, Chang SM, Yung WKA, Drappatz J, Mehta MP, Levin VA, Aldape K, Dancey JE, Wright JJ, Prados MD, Cloughesy TF, Wen PY, Gilbert MR. Phase I/II study of sorafenib in combination with erlotinib for recurrent glioblastoma as part of a 3-arm sequential accrual clinical trial: NABTC 05-02. Neurooncol Adv. 2020 JAN 01; 2(1):vdaa124. DOI: 10.1093/noajnl/vdaa124. 2020 Sep 17. PubMed PMID: 33235994; PMCID: PMC7668489. |
| McHugh D, Eisenberger M, Heath EI, Bruce J, Danila DC, Rathkopf DE, Feldman J, Slovin SF, Anand B, Chu R, Lackey J, Reyno L, Antonarakis ES, Morris MJ. A phase I study of the antibody drug conjugate ASG-5ME, an SLC44A4-targeting antibody carrying auristatin E, in metastatic castration-resistant prostate cancer. Invest New Drugs. 2019 OCT 01; 37(5):1052-1060. DOI: 10.1007/s10637-019-00731-5. 2019 Feb 6. PubMed PMID: 30725389; PMCID: PMC6684870. |
| Heath E, Heilbrun L, Mannuel H, Liu G, Lara P, Monk JP, Flaig T, Zurita A, Mack P, Vaishampayan U, Stella P, Smith D, Bolton S, Hussain A, Al-Janadi A, Silbiger D, Usman M, Ivy SP. Phase II, Multicenter, Randomized Trial of Docetaxel plus Prednisone with or Without Cediranib in Men with Chemotherapy-Naive Metastatic Castrate-Resistant Prostate Cancer. Oncologist. 2019 SEP 01; 24(9):1149-e807. DOI: 10.1634/theoncologist.2019-0331. 2019 May 31. PubMed PMID: 31152080; PMCID: PMC6738301. |
| Michaelson MD, Gupta S, Agarwal N, Szmulewitz R, Powles T, Pili R, Bruce JY, Vaishampayan U, Larkin J, Rosbrook B, Wang E, Murphy D, Wang P, Lechuga MJ, Valota O, Shepard DR. A Phase Ib Study of Axitinib in Combination with Crizotinib in Patients with Metastatic Renal Cell Cancer or Other Advanced Solid Tumors. Oncologist. 2019 SEP 01; 24(9):1151-e817. DOI: 10.1634/theoncologist.2018-0749. 2019 Jun 6. PubMed PMID: 31171735; PMCID: PMC6738313. |
| Tarhini AA, Lee SJ, Li X, Rao UNM, Nagarajan A, Albertini MR, Mitchell JW, Wong SJ, Taylor MA, Laudi N, Truong PV, Conry RM, Kirkwood JM. E3611-A Randomized Phase II Study of Ipilimumab at 3 or 10 mg/kg Alone or in Combination with High-Dose Interferon-α2b in Advanced Melanoma. Clin. Cancer Res.. 2019 JAN 15; 25(2):524-532. DOI: 10.1158/1078-0432.CCR-18-2258. 2018 Nov 12. PubMed PMID: 30420448; PMCID: PMC6335150. |
| Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, Jordan RCK, Zhao W, Sturgis EM, Burtness B, Ridge JA, Ringash J, Galvin J, Yao M, Koyfman SA, Blakaj DM, Razaq MA, Colevas AD, Beitler JJ, Jones CU, Dunlap NE, Seaward SA, Spencer S, Galloway TJ, Phan J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019 JAN 05; 393(10166):40-50. DOI: 10.1016/S0140-6736(18)32779-X. 2018 Nov 15. PubMed PMID: 30449625; PMCID: PMC6541928. |
| Zhang QE, Wu Q, Harari PM, Rosenthal DI. Randomized phase II/III confirmatory treatment selection design with a change of survival end points: Statistical design of Radiation Therapy Oncology Group 1216. Head Neck. 2019 JAN 01; 41(1):37-45. DOI: 10.1002/hed.25359. 2018 Dec 14. PubMed PMID: 30549358; PMCID: PMC6587571. |
| Gorbunova V, Beck JT, Hofheinz RD, Garcia-Alfonso P, Nechaeva M, Cubillo Gracian A, Mangel L, Elez Fernandez E, Deming DA, Ramanathan RK, Torres AH, Sullivan D, Luo Y, Berlin JD. A phase 2 randomised study of veliparib plus FOLFIRI±bevacizumab versus placebo plus FOLFIRI±bevacizumab in metastatic colorectal cancer. Br. J. Cancer. 2019 JAN 01; 120(2):183-189. DOI: 10.1038/s41416-018-0343-z. 2018 Dec 11. PubMed PMID: 30531832; PMCID: PMC6342906. |
| Sahai V, Catalano PJ, Zalupski MM, Lubner SJ, Menge MR, Nimeiri HS, Munshi HG, Benson AB, O’Dwyer PJ. Nab-Paclitaxel and Gemcitabine as First-line Treatment of Advanced or Metastatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018 DEC 01; 4(12):1707-1712. DOI: 10.1001/jamaoncol.2018.3277. PubMed PMID: 30178032; PMCID: PMC6440720. |
| Straus DJ, Jung SH, Pitcher B, Kostakoglu L, Grecula JC, Hsi ED, Schöder H, Popplewell LL, Chang JE, Moskowitz CH, Wagner-Johnston N, Leonard JP, Friedberg JW, Kahl BS, Cheson BD, Bartlett NL. CALGB 50604: risk-adapted treatment of nonbulky early-stage Hodgkin lymphoma based on interim PET. Blood. 2018 SEP 06; 132(10):1013-1021. DOI: 10.1182/blood-2018-01-827246. 2018 Jul 26. PubMed PMID: 30049811; PMCID: PMC6128083. |
| Miller KD, O’Neill A, Gradishar W, Hobday TJ, Goldstein LJ, Mayer IA, Bloom S, Brufsky AM, Tevaarwerk AJ, Sparano JA, Le-Lindqwister NA, Hendricks CB, Northfelt DW, Dang CT, Sledge GW. Double-Blind Phase III Trial of Adjuvant Chemotherapy With and Without Bevacizumab in Patients With Lymph Node-Positive and High-Risk Lymph Node-Negative Breast Cancer (E5103). J. Clin. Oncol.. 2018 SEP 01; 36(25):2621-2629. DOI: 10.1200/JCO.2018.79.2028. 2018 Jul 24. PubMed PMID: 30040523; PMCID: PMC6118403. |
| Byrd JC, Ruppert AS, Heerema NA, Halvorson AE, Hoke E, Smith MR, Godwin JE, Couban S, Fehniger TA, Thirman MJ, Tallman MS, Appelbaum FR, Stone RM, Robinson S, Chang JE, Mandrekar SJ, Larson RA. Lenalidomide consolidation benefits patients with CLL receiving chemoimmunotherapy: results for CALGB 10404 (Alliance). Blood Adv. 2018 JUL 24; 2(14):1705-1718. DOI: 10.1182/bloodadvances.2017015396. PubMed PMID: 30030269; PMCID: PMC6058242. |
| Horn L, Infante JR, Reckamp KL, Blumenschein GR, Leal TA, Waqar SN, Gitlitz BJ, Sanborn RE, Whisenant JG, Du L, Neal JW, Gockerman JP, Dukart G, Harrow K, Liang C, Gibbons JJ, Holzhausen A, Lovly CM, Wakelee HA. Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results from a First-in-Human Phase I/II, Multicenter Study. Clin. Cancer Res.. 2018 JUN 15; 24(12):2771-2779. DOI: 10.1158/1078-0432.CCR-17-2398. 2018 Mar 21. PubMed PMID: 29563138; PMCID: PMC6004248. |
| Miller JS, Morishima C, McNeel DG, Patel MR, Kohrt HEK, Thompson JA, Sondel PM, Wakelee HA, Disis ML, Kaiser JC, Cheever MA, Streicher H, Creekmore SP, Waldmann TA, Conlon KC. A First-in-Human Phase I Study of Subcutaneous Outpatient Recombinant Human IL15 (rhIL15) in Adults with Advanced Solid Tumors. Clin. Cancer Res.. 2018 APR 01; 24(7):1525-1535. DOI: 10.1158/1078-0432.CCR-17-2451. 2017 Dec 4. PubMed PMID: 29203590; PMCID: PMC6741437. |
| Yeruva SLH, Zhao F, Miller KD, Tevaarwerk AJ, Wagner LI, Gray RJ, Sparano JA, Connolly RM. E2112: randomized phase iii trial of endocrine therapy plus entinostat/placebo in patients with hormone receptor-positive advanced breast cancer. NPJ Breast Cancer. 2018 JAN 01; 4:1. DOI: 10.1038/s41523-017-0053-3. 2018 Jan 11. PubMed PMID: 29354686; PMCID: PMC5765007. |
| Nghiemphu PL, Ebiana VA, Wen P, Gilbert M, Abrey LE, Lieberman F, DeAngelis LM, Robins HI, Yung WKA, Chang S, Drappatz J, Mehta MP, Levin VA, Aldape K, Dancey JE, Wright JJ, Prados M, Kuhn J, Cloughesy TF. Phase I study of sorafenib and tipifarnib for recurrent glioblastoma: NABTC 05-02. J. Neurooncol.. 2018 JAN 01; 136(1):79-86. DOI: 10.1007/s11060-017-2624-4. 2017 Oct 7. PubMed PMID: 28988377; PMCID: PMC5756101. |
| Wakelee HA, Dahlberg SE, Keller SM, Tester WJ, Gandara DR, Graziano SL, Adjei AA, Leighl NB, Aisner SC, Rothman JM, Patel JD, Sborov MD, McDermott SR, Perez-Soler R, Traynor AM, Butts C, Evans T, Shafqat A, Chapman AE, Kasbari SS, Horn L, Ramalingam SS, Schiller JH. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol.. 2017 DEC 01; 18(12):1610-1623. DOI: 10.1016/S1470-2045(17)30691-5. 2017 Nov 9. PubMed PMID: 29129443; PMCID: PMC5789803. |
| Pili R, Jegede O, Carducci MA, Manola J, Groteluschen DL, Appleman LL, Liu G, Shanks JC, Dakhil SR, Dutcher J, DiPaola RS. A Randomized Phase II Study to Determine the Effect of 2 Different Doses of Aflibercept in Patients With Metastatic Renal Cell Carcinoma (ECOG-ACRIN [E4805]). Clin Genitourin Cancer. 2017 DEC 01; 15(6):642-651.e1. DOI: 10.1016/j.clgc.2017.04.023. 2017 Apr 26. PubMed PMID: 28545998; PMCID: PMC5675826. |
| Ma CX, Suman V, Goetz MP, Northfelt D, Burkard ME, Ademuyiwa F, Naughton M, Margenthaler J, Aft R, Gray R, Tevaarwerk AJ, Wilke LG, Haddad T, Moynihan T, Loprinzi C, Hieken T, Barnell EK, Skidmore ZL, Feng YY, Krysiak K, Hoog J, Guo Z, Nehring L, Wisinski KB, Mardis E, et al. A Phase II Trial of Neoadjuvant MK-2206, an AKT Inhibitor, with Anastrozole in Clinical Stage II or III PIK3CA-Mutant ER-Positive and HER2-Negative Breast Cancer. Clin. Cancer Res.. 2017 NOV 15; 23(22):6823-6832. DOI: 10.1158/1078-0432.CCR-17-1260. 2017 Sep 5. PubMed PMID: 28874413; PMCID: PMC6392430. |
| Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O’Neil BH, Atkins JN, Berry S, Polite BN, O’Reilly EM, Goldberg RM, Hochster HS, Schilsky RL, Bertagnolli MM, El-Khoueiry AB, Watson P, Benson AB, Mulkerin DL, Mayer RJ, Blanke C. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA. 2017 JUN 20; 317(23):2392-2401. DOI: 10.1001/jama.2017.7105. PubMed PMID: 28632865; PMCID: PMC5545896. |
| Czito BG, Deming DA, Jameson GS, Mulcahy MF, Vaghefi H, Dudley MW, Holen KD, DeLuca A, Mittapalli RK, Munasinghe W, He L, Zalcberg JR, Ngan SY, Komarnitsky P, Michael M. Safety and tolerability of veliparib combined with capecitabine plus radiotherapy in patients with locally advanced rectal cancer: a phase 1b study. Lancet Gastroenterol Hepatol. 2017 JUN 01; 2(6):418-426. DOI: 10.1016/S2468-1253(17)30012-2. 2017 Mar 27. PubMed PMID: 28497757. |
| Morris VK, Salem ME, Nimeiri H, Iqbal S, Singh P, Ciombor K, Polite B, Deming D, Chan E, Wade JL, Xiao L, Bekaii-Saab T, Vence L, Blando J, Mahvash A, Foo WC, Ohaji C, Pasia M, Bland G, Ohinata A, Rogers J, Mehdizadeh A, Banks K, Lanman R, Wolff RA, et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol.. 2017 APR 01; 18(4):446-453. DOI: 10.1016/S1470-2045(17)30104-3. 2017 Feb 18. PubMed PMID: 28223062; PMCID: PMC5809128. |
| Agarwala SS, Lee SJ, Yip W, Rao UN, Tarhini AA, Cohen GI, Reintgen DS, Evans TL, Brell JM, Albertini MR, Atkins MB, Dakhil SR, Conry RM, Sosman JA, Flaherty LE, Sondak VK, Carson WE, Smylie MG, Pappo AS, Kefford RF, Kirkwood JM. Phase III Randomized Study of 4 Weeks of High-Dose Interferon-α-2b in Stage T2bNO, T3a-bNO, T4a-bNO, and T1-4N1a-2a (microscopic) Melanoma: A Trial of the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (E1697). J. Clin. Oncol.. 2017 MAR 10; 35(8):885-892. DOI: 10.1200/JCO.2016.70.2951. 2017 Jan 30. PubMed PMID: 28135150; PMCID: PMC5455684. |
| Chang S, Zhang P, Cairncross JG, Gilbert MR, Bahary JP, Dolinskas CA, Chakravarti A, Aldape KD, Bell EH, Schiff D, Jaeckle K, Brown PD, Barger GR, Werner-Wasik M, Shih H, Brachman D, Penas-Prado M, Robins HI, Belanger K, Schultz C, Hunter G, Mehta M. Phase III randomized study of radiation and temozolomide versus radiation and nitrosourea therapy for anaplastic astrocytoma: results of NRG Oncology RTOG 9813. Neuro-oncology. 2017 FEB 01; 19(2):252-258. DOI: 10.1093/neuonc/now236. PubMed PMID: 27994066; PMCID: PMC5463834. |
| Older Tomoda K, Tam YT, Cho H, Buehler D, Kozak KR, Kwon GS. Triolimus: A Multi-Drug Loaded Polymeric Micelle Containing Paclitaxel, 17-AAG, and Rapamycin as a Novel Radiosensitizer. Macromolecular bioscience. 2017;17(1). Epub 2016/07/02. doi: 10.1002/mabi.201600194. PubMed PMID: 27365266; PMCID: PMC5320892.Bruce JY, LoRusso PM, Goncalves PH, Heath EI, Sadowski E, Shalinsky DR, Zhang Y, Traynor AM, Breazna A, Ricart AD, Tortorici M, Liu G. A pharmacodynamically guided dose selection of PF-00337210 in a phase I study in patients with advanced solid tumors. Cancer chemotherapy and pharmacology. 2016;77(3):527-38. Epub 2016/01/23. doi: 10.1007/s00280-016-2958-1. PubMed PMID: 26791870. Deming DA, Cavalcante LL, Lubner SJ, Mulkerin DL, LoConte NK, Eickhoff JC, Kolesar JM, Fioravanti S, Greten TF, Compton K, Doyle AG, Wilding G, Duffy A, Liu G. A phase I study of selumetinib (AZD6244/ARRY-142866), a MEK1/2 inhibitor, in combination with cetuximab in refractory solid tumors and KRAS mutant colorectal cancer. Investigational new drugs. 2016;34(2):168-75. Epub 2015/12/17. doi: 10.1007/s10637-015-0314-7. PubMed PMID: 26666244; PMCID: PMC4788533. Gee JR, Saltzstein DR, Messing E, Kim K, Kolesar J, Huang W, Havighurst TC, Harris L, Wollmer BW, Jarrard D, House M, Parnes H, Bailey HH. Phase Ib placebo-controlled, tissue biomarker trial of diindolylmethane (BR-DIMNG) in patients with prostate cancer who are undergoing prostatectomy. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP). 2016;25(4):312-20. Epub 2015/08/28. doi: 10.1097/cej.0000000000000189. PubMed PMID: 26313229. Ivashko IN, Kolesar JM. Pembrolizumab and nivolumab: PD-1 inhibitors for advanced melanoma. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2016;73(4):193-201. Epub 2016/02/05. doi: 10.2146/ajhp140768. PubMed PMID: 26843495. Jarrard D, Konety B, Huang W, Downs T, Kolesar J, Kim KM, Havighurst T, Slaton J, House MG, Parnes HL, Bailey HH. Phase IIa, randomized placebo-controlled trial of single high dose cholecalciferol (vitamin D3) and daily Genistein (G-2535) versus double placebo in men with early stage prostate cancer undergoing prostatectomy. American journal of clinical and experimental urology. 2016;4(2):17-27. Epub 2016/10/22. PubMed PMID: 27766277; PMCID: PMC5069272. Kujak C, Kolesar JM. Treatment of chronic myelogenous leukemia. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2016;73(3):113-20. Epub 2016/01/23. doi: 10.2146/ajhp140686. PubMed PMID: 26796903. Kyriakopoulos CE, Braden AM, Kolesar JM, Eickhoff JC, Bailey HH, Heideman J, Liu G, Wisinski KB. A phase I study of tivantinib in combination with temsirolimus in patients with advanced solid tumors. Investigational new drugs. 2016. Epub 2016/12/23. doi: 10.1007/s10637-016-0418-8. PubMed PMID: 28004284. Kyriakopoulos CE, Heath EI, Eickhoff JC, Kolesar J, Yayehyirad M, Moll T, Wilding G, Liu G. A multicenter phase 1/2a dose-escalation study of the antioxidant moiety of vitamin E 2,2,5,7,8-pentamethyl-6-chromanol (APC-100) in men with advanced prostate cancer. Investigational new drugs. 2016;34(2):225-30. Epub 2016/03/01. doi: 10.1007/s10637-016-0334-y. PubMed PMID: 26924129. Munroe M, Kolesar J. Olaparib for the treatment of BRCA-mutated advanced ovarian cancer. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2016;73(14):1037-41. Epub 2016/07/08. doi: 10.2146/ajhp150550. PubMed PMID: 27385701. Scarpelli M, Bruce JY, Carmichael L, Eickhoff J, Kolesar J, Perlman S, Jeraj R, Liu G. 18F-FLT PET/CT imaging in patients with advanced solid malignancies treated with axitinib on an intermittent dosing regimen. Cancer chemotherapy and pharmacology. 2016;78(6):1245-52. Epub 2016/11/07. doi: 10.1007/s00280-016-3183-7. PubMed PMID: 27817059; PMCID: PMC5209788. Sippy R, Kolesar JM, Darst BF, Engelman CD. Prioritization of family member sequencing for the detection of rare variants. BMC proceedings. 2016;10(Suppl 7):227-31. Epub 2016/12/17. doi: 10.1186/s12919-016-0035-8. PubMed PMID: 27980641; PMCID: PMC5133500. Walko C, Kiel PJ, Kolesar J. Precision medicine in oncology: New practice models and roles for oncology pharmacists. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2016;73(23):1935-42. Epub 2016/11/20. doi: 10.2146/ajhp160211. PubMed PMID: 27864201. Wisinski KB, Tevaarwerk AJ, Burkard ME, Rampurwala M, Eickhoff J, Bell MC, Kolesar JM, Flynn C, Liu G. Phase I Study of an AKT Inhibitor (MK-2206) Combined with Lapatinib in Adult Solid Tumors Followed by Dose Expansion in Advanced HER2+ Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(11):2659-67. Epub 2016/03/31. doi: 10.1158/1078-0432.ccr-15-2365. PubMed PMID: 27026198; PMCID: PMC4891227. Bruce JY, Scully PC, Carmichael LL, Eickhoff JC, Perlman SB, Kolesar JM, Heideman JL, Jeraj R, Liu G. Pharmacodynamic study of axitinib in patients with advanced malignancies assessed with (18)F-3’deoxy-3’fluoro-L-thymidine positron emission tomography/computed tomography. Cancer chemotherapy and pharmacology. 2015;76(1):187-95. Epub 2015/05/30. doi: 10.1007/s00280-015-2779-7. PubMed PMID: 26021741; PMCID: PMC4499265. Croegaert K, Kolesar JM. Role of anaplastic lymphoma kinase inhibition in the treatment of non-small-cell lung cancer. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2015;72(17):1456-62. Epub 2015/08/22. doi: 10.2146/ajhp140836. PubMed PMID: 26294238. Deming D, Uboha N, Zafar SY, Rosenberg S, Bassetti M, Glasgow S, Borden EC, Lubner S. Adjuvant Chemotherapy for Stage II Rectal Cancer. Seminars in oncology. 2015;42(6):e99-107. Epub 2015/11/29. doi: 10.1053/j.seminoncol.2015.09.033. PubMed PMID: 26615141. Engle JA, Kolesar JM. Importance of ethnicity and smoking status in EGFR gene testing in lung cancers. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2015;72(9):686-7. Epub 2015/04/16. doi: 10.2146/ajhp150001. PubMed PMID: 25873612. Hanna NH, Dahlberg SE, Kolesar JM, Aggarwal C, Hirsch FR, Ramalingam SS, Schiller JH. Three-arm, randomized, phase 2 study of carboplatin and paclitaxel in combination with cetuximab, cixutumumab, or both for advanced non-small cell lung cancer (NSCLC) patients who will not receive bevacizumab-based therapy: An Eastern Cooperative Oncology Group (ECOG) study (E4508). Cancer. 2015;121(13):2253-61. Epub 2015/03/06. doi: 10.1002/cncr.29308. PubMed PMID: 25740387; PMCID: PMC4560671. Kolesar JM, Pomplun M, Havighurst T, Stublaski J, Wollmer B, Kim K, Tangrea JA, Parnes HL, House MG, Gee J, Messing E, Bailey HH. Soy food frequency questionnaire does not correlate with baseline isoflavone levels in patients with bladder cancer. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2015;21(2):128-31. Epub 2014/03/20. doi: 10.1177/1078155214528552. PubMed PMID: 24642450; PMCID: PMC4261043. LoConte NK, Razak AR, Ivy P, Tevaarwerk A, Leverence R, Kolesar J, Siu L, Lubner SJ, Mulkerin DL, Schelman WR, Deming DA, Holen KD, Carmichael L, Eickhoff J, Liu G. A multicenter phase 1 study of gamma -secretase inhibitor RO4929097 in combination with capecitabine in refractory solid tumors. Investigational new drugs. 2015;33(1):169-76. Epub 2014/10/17. doi: 10.1007/s10637-014-0166-6. PubMed PMID: 25318436; PMCID: PMC4297251. Rastegar-Mojarad M, Ye Z, Kolesar JM, Hebbring SJ, Lin SM. Opportunities for drug repositioning from phenome-wide association studies. Nature biotechnology. 2015;33(4):342-5. Epub 2015/04/08. doi: 10.1038/nbt.3183. PubMed PMID: 25850054. Rounds A, Kolesar J. Nivolumab for second-line treatment of metastatic squamous non-small-cell lung cancer. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2015;72(21):1851-5. Epub 2015/10/23. doi: 10.2146/ajhp150235. PubMed PMID: 26490818. Tran NH, Cavalcante LL, Lubner SJ, Mulkerin DL, LoConte NK, Clipson L, Matkowskyj KA, Deming DA. Precision medicine in colorectal cancer: the molecular profile alters treatment strategies. Therapeutic advances in medical oncology. 2015;7(5):252-62. Epub 2015/09/04. doi: 10.1177/1758834015591952. PubMed PMID: 26327923; PMCID: PMC4543854. Wisinski KB, Cantu CA, Eickhoff J, Osterby K, Tevaarwerk AJ, Heideman J, Liu G, Wilding G, Johnston S, Kolesar JM. Potential cytochrome P-450 drug-drug interactions in adults with metastatic solid tumors and effect on eligibility for Phase I clinical trials. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2015;72(11):958-65. Epub 2015/05/20. doi: 10.2146/ajhp140591. PubMed PMID: 25987691; PMCID: PMC4510955. Wisinski KB, Ledesma WM, Kolesar J, Wilding G, Liu G, Douglas J, Traynor AM, Albertini M, Mulkerin D, Bailey HH. A phase I study to determine the maximum tolerated dose and safety of oral LR-103 (1alpha,24(S)Dihydroxyvitamin D2) in patients with advanced cancer. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2015;21(6):416-24. Epub 2014/07/06. doi: 10.1177/1078155214541572. PubMed PMID: 24986793; PMCID: PMC4281509. Bruce JY, Kolesar JM, Hammers H, Stein MN, Carmichael L, Eickhoff J, Johnston SA, Binger KA, Heideman JL, Perlman SB, Jeraj R, Liu G. A phase I pharmacodynamic trial of sequential sunitinib with bevacizumab in patients with renal cell carcinoma and other advanced solid malignancies. Cancer chemotherapy and pharmacology. 2014;73(3):485-93. Epub 2014/01/15. doi: 10.1007/s00280-013-2373-9. PubMed PMID: 24414551; PMCID: PMC4200479. Deming DA, Ninan J, Bailey HH, Kolesar JM, Eickhoff J, Reid JM, Ames MM, McGovern RM, Alberti D, Marnocha R, Espinoza-Delgado I, Wright J, Wilding G, Schelman WR. A Phase I study of intermittently dosed vorinostat in combination with bortezomib in patients with advanced solid tumors. Investigational new drugs. 2014;32(2):323-9. Epub 2013/10/12. doi: 10.1007/s10637-013-0035-8. PubMed PMID: 24114123; PMCID: PMC3949160. Engle JA, Kolesar JM. Afatinib: A first-line treatment for selected patients with metastatic non-small-cell lung cancer. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2014;71(22):1933-8. Epub 2014/10/29. doi: 10.2146/ajhp130654. PubMed PMID: 25349236. Grudzinski JJ, Titz B, Kozak K, Clarke W, Allen E, Trembath L, Stabin M, Marshall J, Cho SY, Wong TZ, Mortimer J, Weichert JP. A phase 1 study of 131I-CLR1404 in patients with relapsed or refractory advanced solid tumors: dosimetry, biodistribution, pharmacokinetics, and safety. PloS one. 2014;9(11):e111652. Epub 2014/11/18. doi: 10.1371/journal.pone.0111652. PubMed PMID: 25402488; PMCID: PMC4234270. Kirschling RJ, Johnson PH, Huie MS, Wims ME, Larson MM, Hernan HR, Traynor AM. Vorinostat and bortezomib as third-line therapy in patients with advanced non-small cell lung cancer: a Wisconsin Oncology Network Phase II study. Investigational new drugs. 2014;32(1):195-9. Epub 2013/06/04. doi: 10.1007/s10637-013-9980-5. PubMed PMID: 23728919; PMCID: PMC4310688. Kolesar JM, Eickhoff J, Vermeulen LC. Serotonin type 3-receptor antagonists for chemotherapy-induced nausea and vomiting: therapeutically equivalent or meaningfully different? American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2014;71(6):507-10. Epub 2014/03/05. doi: 10.2146/ajhp130653. PubMed PMID: 24589542; PMCID: PMC4158452. Piccolo J, Kolesar JM. Prevention and treatment of chemotherapy-induced peripheral neuropathy. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2014;71(1):19-25. Epub 2013/12/20. doi: 10.2146/ajhp130126. PubMed PMID: 24352178. Schelman WR, Mohammed TA, Traynor AM, Kolesar JM, Marnocha RM, Eickhoff J, Keppen M, Alberti DB, Wilding G, Takebe N, Liu G. A phase I study of AT-101 with cisplatin and etoposide in patients with advanced solid tumors with an expanded cohort in extensive-stage small cell lung cancer. Investigational new drugs. 2014;32(2):295-302. Epub 2013/07/19. doi: 10.1007/s10637-013-9999-7. PubMed PMID: 23860642; PMCID: PMC3895103. Zasadil LM, Andersen KA, Yeum D, Rocque GB, Wilke LG, Tevaarwerk AJ, Raines RT, Burkard ME, Weaver BA. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Science translational medicine. 2014;6(229):229ra43. Epub 2014/03/29. doi: 10.1126/scitranslmed.3007965. PubMed PMID: 24670687; PMCID: PMC4176609. Argiris A, Ghebremichael M, Gilbert J, Lee JW, Sachidanandam K, Kolesar JM, Burtness B, Forastiere AA. Phase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: an eastern cooperative oncology group trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(11):1405-14. Epub 2013/03/06. doi: 10.1200/jco.2012.45.4272. PubMed PMID: 23460714; PMCID: PMC3612594. Banaszynski M, Kolesar JM. Vemurafenib and ipilimumab: new agents for metastatic melanoma. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2013;70(14):1205-10. Epub 2013/07/04. doi: 10.2146/ajhp120260. PubMed PMID: 23820456. Gee J, Bailey H, Kim K, Kolesar J, Havighurst T, Tutsch KD, See W, Cohen MB, Street N, Levan L, Jarrard D, Wilding G. Phase II open label, multi-center clinical trial of modulation of intermediate endpoint biomarkers by 1alpha-hydroxyvitamin D2 in patients with clinically localized prostate cancer and high grade pin. The Prostate. 2013;73(9):970-8. Epub 2013/01/22. doi: 10.1002/pros.22644. PubMed PMID: 23335089; PMCID: PMC3755376. Greenwald DR, Li H, Luger SM, Go RS, King D, Patel T, Gascoyne RD, Kolesar J, Kahl BS, Horning S. A phase II study of sorafenib (BAY 43-9006) in recurrent diffuse large B cell lymphoma: an eastern cooperative oncology group study (E1404). Journal of hematology & oncology. 2013;6:46. Epub 2013/07/09. doi: 10.1186/1756-8722-6-46. PubMed PMID: 23829878; PMCID: PMC3716977. Kolesar JM, Traynor AM, Holen KD, Hoang T, Seo S, Kim K, Alberti D, Espinoza-Delgado I, Wright JJ, Wilding G, Bailey HH, Schelman WR. Vorinostat in combination with bortezomib in patients with advanced malignancies directly alters transcription of target genes. Cancer chemotherapy and pharmacology. 2013;72(3):661-7. Epub 2013/08/02. doi: 10.1007/s00280-013-2242-6. PubMed PMID: 23903894; PMCID: PMC3926898. Kolesar JM, Vermeulen L. Assays for biological agents. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2013;70(13):1101. Epub 2013/06/21. doi: 10.2146/ajhp130128. PubMed PMID: 23784155. Liu G, Chen YH, Kolesar J, Huang W, Dipaola R, Pins M, Carducci M, Stein M, Bubley GJ, Wilding G. Eastern Cooperative Oncology Group Phase II Trial of lapatinib in men with biochemically relapsed, androgen dependent prostate cancer. Urologic oncology. 2013;31(2):211-8. Epub 2011/07/26. doi: 10.1016/j.urolonc.2011.01.002. PubMed PMID: 21784672; PMCID: PMC3223557. LoConte NK, Holen KD, Schelman WR, Mulkerin DL, Deming DA, Hernan HR, Traynor AM, Goggins T, Groteluschen D, Oettel K, Robinson E, Lubner SJ. A phase I study of sorafenib, oxaliplatin and 2 days of high dose capecitabine in advanced pancreatic and biliary tract cancer: a Wisconsin oncology network study. Investigational new drugs. 2013;31(4):943-8. Epub 2012/12/25. doi: 10.1007/s10637-012-9916-5. PubMed PMID: 23263993; PMCID: PMC4199231. Poggi L, Kolesar JM. Vismodegib for the treatment of basal cell skin cancer. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2013;70(12):1033-8. Epub 2013/05/31. doi: 10.2146/ajhp120311. PubMed PMID: 23719880. Sachidanandam K, Gayle AA, Robins HI, Kolesar JM. Unexpected doxorubicin-mediated cardiotoxicity in sisters: possible role of polymorphisms in histamine n-methyl transferase. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2013;19(3):269-72. Epub 2012/11/17. doi: 10.1177/1078155212461022. PubMed PMID: 23154571; PMCID: PMC3998823. Simondsen K, Kolesar J. New treatment options for castration-resistant prostate cancer. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2013;70(10):856-65. Epub 2013/05/04. doi: 10.2146/ajhp110586. PubMed PMID: 23640346. Timm A, Kolesar JM. Crizotinib for the treatment of non-small-cell lung cancer. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2013;70(11):943-7. Epub 2013/05/21. doi: 10.2146/ajhp120261. PubMed PMID: 23686600. Ton GN, Banaszynski ME, Kolesar JM. Vandetanib: a novel targeted therapy for the treatment of metastatic or locally advanced medullary thyroid cancer. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2013;70(10):849-55. Epub 2013/05/04. doi: 10.2146/ajhp120253. PubMed PMID: 23640345. Zhang Y, Vermeulen LC, Kolesar JM. Stability of stock and diluted rituximab. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2013;70(5):436-8. Epub 2013/02/16. doi: 10.2146/ajhp120035. PubMed PMID: 23413167; PMCID: PMC3987107. Gorden KJ, Mesbah P, Kolesar JM. EGFR inhibitors as first-line therapy in EGFR mutation-positive patients with NSCLC. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2012;18(2):245-9. Epub 2011/07/08. doi: 10.1177/1078155211408373. PubMed PMID: 21733906. Hoang T, Campbell TC, Zhang C, Kim K, Kolesar JM, Oettel KR, Blank JH, Robinson EG, Ahuja HG, Ikeda R, Vermeulen LC, Lau E, Jiang Z, Saha S, Reichelderfer M, Kolesar JM. Stability of infliximab in polyvinyl chloride bags. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2012;69(17):1509-12. Epub 2012/08/18. doi: 10.2146/ajhp100116. PubMed PMID: 22899746; PMCID: PMC3987119. Messing E, Gee JR, Saltzstein DR, Kim K, diSant’Agnese A, Kolesar J, Harris L, Faerber A, Havighurst T, Young JM, Efros M, Getzenberg RH, Wheeler MA, Tangrea J, Parnes H, House M, Busby JE, Hohl R, Bailey H. A phase 2 cancer chemoprevention biomarker trial of isoflavone G-2535 (genistein) in presurgical bladder cancer patients. Cancer prevention research (Philadelphia, Pa). 2012;5(4):621-30. Epub 2012/02/02. doi: 10.1158/1940-6207.capr-11-0455. PubMed PMID: 22293631; PMCID: PMC3324663. Pearson R, Kolesar JM. Targeted therapy for NSCLC: ALK inhibition. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2012;18(2):271-4. Epub 2011/08/17. doi: 10.1177/1078155211417477. PubMed PMID: 21844131. Richardson PG, Eng C, Kolesar J, Hideshima T, Anderson KC. Perifosine , an oral, anti-cancer agent and inhibitor of the Akt pathway: mechanistic actions, pharmacodynamics, pharmacokinetics, and clinical activity. Expert opinion on drug metabolism & toxicology. 2012;8(5):623-33. Epub 2012/04/20. doi: 10.1517/17425255.2012.681376. PubMed PMID: 22512706; PMCID: PMC4467022. Shin HC, Cho H, Lai TC, Kozak KR, Kolesar JM, Kwon GS. Pharmacokinetic study of 3-in-1 poly(ethylene glycol)-block-poly(D, L-lactic acid) micelles carrying paclitaxel, 17-allylamino-17-demethoxygeldanamycin, and rapamycin. Journal of controlled release : official journal of the Controlled Release Society. 2012;163(1):93-9. Epub 2012/05/03. doi: 10.1016/j.jconrel.2012.04.024. PubMed PMID: 22549011; PMCID: PMC3422612. Simondsen KA, Kolesar JM. Lenalidomide-induced elevated bilirubin. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2012;18(4):402-5. Epub 2012/03/13. doi: 10.1177/1078155212439492. PubMed PMID: 22407059. Zhang Y, Simondsen K, Kolesar JM. Exemestane for primary prevention of breast cancer in postmenopausal women. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2012;69(16):1384-8. Epub 2012/08/03. doi: 10.2146/ajhp110585. PubMed PMID: 22855103. Ramalingam SS, Lee JW, Belani CP, Aisner SC, Kolesar J, Howe C, Velasco MR, Schiller JH. Cetuximab for the treatment of advanced bronchioloalveolar carcinoma (BAC): an Eastern Cooperative Oncology Group phase II study (ECOG 1504). J Clin Oncol. 2011;29(13):1709-14. PMCID: PMC3107763. |
| Liu G, Jeraj R, Vanderhoek M, Perlman S, Kolesar J, Harrison M, Simoncic U, Eickhoff J, Carmichael L, Chao B, Marnocha R, Ivy P, Wilding G. Pharmacodynamic study using FLT PET/CT in patients with renal cell cancer and other solid malignancies treated with sunitinib malate. Clin Cancer Res. 2011;17(24):7634-44. PMCID: PMC3243764. |
| Dennie TW, Fleming RA, Bowen CJ, Dar MM, Alberti D, Oliver K, Loconte N, Mulkerin D, Holen KD. A phase I study of capecitabine, oxaliplatin, and lapatinib in metastatic or advanced solid tumors. Clin Colorectal Cancer. 2011;10(1):57-62. |
| Tevaarwerk A, Wilding G, Eickhoff J, Chappell R, Sidor C, Arnott J, Bailey H, Schelman W, Liu G. Phase I study of continuous MKC-1 in patients with advanced or metastatic solid malignancies using the modified Time-to-Event Continual Reassessment Method (TITE-CRM) dose escalation design. Invest New Drugs. 2011. PMCID: PMC3139017. |
| Cetnar J, Wilding G, McNeel D, Loconte NK, McFarland TA, Eickhoff J, Liu G. A phase 1/1b study of satraplatin (JM-216) in combination with docetaxel in patients with advanced solid tumors and metastatic castrate-resistant prostate cancer. Urol Oncol. 2011. |
| Kolesar JM, Sachidanandam K, Schelman WR, Eickhoff J, Holen KD, Traynor AM, Alberti DB, Thomas JP, Chitambar CR, Wilding G, Antholine WE. Cytotoxic Evaluation of 3-Aminopyridine-2-Carboxaldehyde Thiosemicarbazone, 3-AP, in Peripheral Blood Lymphocytes of Patients with Refractory Solid Tumors using Electron Paramagnetic Resonance. Exp Ther Med. 2011;2(1):119-23. PMCID: PMC3046871. |
| Heath EI, Blumenschein GR, Jr., Cohen RB, Lorusso PM, Loconte NK, Kim ST, Ruiz-Garcia A, Chao RC, Wilding G. Sunitinib in combination with paclitaxel plus carboplatin in patients with advanced solid tumors: phase I study results. Cancer Chemother Pharmacol. 2011;68(3):703-12. |
| Kolesar J, Brundage RC, Pomplun M, Alberti D, Holen K, Traynor A, Ivy P, Wilding G. Population pharmacokinetics of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (Triapine(R)) in cancer patients. Cancer Chemother Pharmacol. 2011;67(2):393-400. PMCID: PMC3059107. |
| Rathkopf D, Liu G, Carducci MA, Eisenberger MA, Anand A, Morris MJ, Slovin SF, Sasaki Y, Takahashi S, Ozono S, Fung NK, Cheng S, Gan J, Gottardis M, Obermeier MT, Reddy J, Zhang S, Vakkalagadda BJ, Alland L, Wilding G, Scher HI, Prostate Cancer Clinical Trials C. Phase I dose-escalation study of the novel antiandrogen BMS-641988 in patients with castration-resistant prostate cancer. Clin Cancer Res. 2011;17(4):880-7. PMCID: PMC3070382. |
| Choi BS, Alberti DB, Schelman WR, Kolesar JM, Thomas JP, Marnocha R, Eickhoff JC, Ivy SP, Wilding G, Holen KD. The maximum tolerated dose and biologic effects of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) in combination with irinotecan for patients with refractory solid tumors. Cancer Chemother Pharmacol. 2010;66(5):973-80. PMCID: PMC2921466. |
| Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, Shimada H, Grupp SA, Seeger R, Reynolds CP, Buxton A, Reisfeld RA, Gillies SD, Cohn SL, Maris JM, Sondel PM, Children’s Oncology Group. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324-34. PMCID: PMC3086629. |
| Wernimont SA, Simonson WT, Greer PA, Seroogy CM, Huttenlocher A. Calpain 4 is not necessary for LFA-1-mediated function in CD4+ T cells. PLoS One. 2010;5(5):e10513. PMCID: PMC2866319. |
| Wang J, Liu Y, Li Z, Du J, Ryu MJ, Taylor PR, Fleming MD, Young KH, Pitot H, Zhang J. Endogenous oncogenic Nras mutation promotes aberrant GM-CSF signaling in granulocytic/monocytic precursors in a murine model of chronic myelomonocytic leukemia. Blood. 2010;116(26):5991-6002. PMCID: PMC3031386. |
| Shusterman S, London WB, Gillies SD, Hank JA, Voss SD, Seeger RC, Reynolds CP, Kimball J, Albertini MR, Wagner B, Gan J, Eickhoff J, DeSantes KB, Cohn SL, Hecht T, Gadbaw B, Reisfeld RA, Maris JM, Sondel PM. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) phase II study. J Clin Oncol. 2010;28(33):4969-75. PMCID: PMC3020698. |
| Lubner SJ, Mahoney MR, Kolesar JL, Loconte NK, Kim GP, Pitot HC, Philip PA, Picus J, Yong WP, Horvath L, Van Hazel G, Erlichman CE, Holen KD. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol. 2010;28(21):3491-7. PMCID: PMC2917213. |
| Kolesar JM, Hoel R, Pomplun M, Havighurst T, Stublaski J, Wollmer B, Krontiras H, Brouillette W, Muccio D, Kim K, Grubbs CJ, Bailey H. A pilot, first-in-human, pharmacokinetic study of 9cUAB30 in healthy volunteers. Cancer Prev Res. 2010;3(12):1565-70. PMCID: PMC3204611. |
| Harzstark AL, Rosenberg JE, Weinberg VK, Sharib J, Ryan CJ, Smith DC, Pagliaro LC, Beer TM, Liu G, Small EJ. Ixabepilone, mitoxantrone, and prednisone for metastatic castration-resistant prostate cancer after docetaxel-based therapy: a phase 2 study of the department of defense prostate cancer clinical trials consortium. Cancer. 2010. |
| Delgado DC, Hank JA, Kolesar J, Lorentzen D, Gan J, Seo S, Kim K, Shusterman S, Gillies SD, Reisfeld RA, Yang R, Gadbaw B, DeSantes KB, London WB, Seeger RC, Maris JM, Sondel PM. Genotypes of NK cell KIR receptors, their ligands, and Fcγ receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res. 2010;70(23):9554-61. PMCID: PMC2999644. |
| Bailey HH, Kim K, Verma AK, Sielaff K, Larson PO, Snow S, Lenaghan T, Viner JL, Douglas J, Dreckschmidt NE, Hamielec M, Pomplun M, Sharata HH, Puchalsky D, Berg ER, Havighurst TC, Carbone PP. A randomized, double-blind, placebo-controlled phase 3 skin cancer prevention study of {alpha}-difluoromethylornithine in subjects with previous history of skin cancer. Cancer Prev Res (Phila). 2010;3(1):35-47. PMCID: PMC2804946. |
| Tevaarwerk AJ, Holen KD, Alberti DB, Sidor C, Arnott J, Quon C, Wilding G, Liu G. Phase I trial of 2-methoxyestradiol NanoCrystal dispersion in advanced solid malignancies. Clin Cancer Res. 2009;15(4):1460-5. PMCID: PMC2892631. |
| Schelman WR, Morgan-Meadows S, Marnocha R, Lee F, Eickhoff J, Huang W, Pomplun M, Jiang Z, Alberti D, Kolesar JM, Ivy P, Wilding G, Traynor AM. A phase I study of Triapine in combination with doxorubicin in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2009;63(6):1147-56. PMCID: PMC3050713. |
| Kolesar J, Huang W, Eickhoff J, Hahn K, Alberti D, Attia S, Schelman W, Holen K, Traynor A, Ivy P, Wilding G. Evaluation of mRNA by Q-RTPCR and protein expression by AQUA of the M2 subunit of ribonucleotide reductase (RRM2) in human tumors. Cancer Chemother Pharmacol. 2009;64(1):79-86. PMCID: PMC3043989. |
| Hank JA, Gan J, Ryu H, Ostendorf A, Stauder MC, Sternberg A, Albertini M, Lo KM, Gillies SD, Eickhoff J, Sondel PM. Immunogenicity of the hu14.18-IL2 immunocytokine molecule in adults with melanoma and children with neuroblastoma. Clin Cancer Res. 2009;15(18):5923-30. PMCID: PMC2745522. |
| Bradley D, Rathkopf D, Dunn R, Stadler WM, Liu G, Smith DC, Pili R, Zwiebel J, Scher H, Hussain M. Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (National Cancer Institute Trial 6862): trial results and interleukin-6 analysis: a study by the Department of Defense Prostate Cancer Clinical Trial Consortium and University of Chicago Phase 2 Consortium. Cancer. 2009;115(23):5541-9. PMCID: PMC2917101. |
| Basu HS, Thompson TA, Church DR, Clower CC, Mehraein-Ghomi F, Amlong CA, Martin CT, Woster PM, Lindstrom MJ, Wilding G. A small molecule polyamine oxidase inhibitor blocks androgen-induced oxidative stress and delays prostate cancer progression in the transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2009;69(19):7689-95. PMCID: PMC2756327. |
| Argiris A, Buchanan A, Brockstein B, Kolesar J, Ghebremichael M, Pins M, Hahn K, Axelrod R, Forastiere A. Docetaxel and irinotecan in recurrent or metastatic head and neck cancer: a phase 2 trial of the Eastern Cooperative Oncology Group. Cancer. 2009;115(19):4504-13. PMCID: PMC2749918. |
| LoConte NK, Thomas JP, Alberti D, Heideman J, Binger K, Marnocha R, Utecht K, Geiger P, Eickhoff J, Wilding G, Kolesar J. A phase I pharmacodynamic trial of bortezomib in combination with doxorubicin in patients with advanced cancer. Cancer Chemother Pharmacol. 2008;63(1):109-15. |
| Attia S, Kolesar J, Mahoney MR, Pitot HC, Laheru D, Heun J, Huang W, Eickhoff J, Erlichman C, Holen KD. A phase 2 consortium (P2C) trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) for advanced adenocarcinoma of the pancreas. Invest New Drugs. 2008;26(4):369-79. |
| Ricart AD, Tolcher AW, Liu G, Holen K, Schwartz G, Albertini M, Weiss G, Yazji S, Ng C, Wilding G. Volociximab, a chimeric monoclonal antibody that specifically binds alpha5beta1 integrin: a phase I, pharmacokinetic, and biological correlative study. Clin Cancer Res. 2008;14(23):7924-9. NIHMSID: NIHMS340779 |
| Liu G, Kolesar J, McNeel DG, Leith C, Schell K, Eickhoff J, Lee F, Traynor A, Marnocha R, Alberti D, Zwiebel J, Wilding G. http://www.ncbi.nlm.nih.gov/pubmed/?term=PMC2950700 target=”_blank” Clin Cancer Res. 2008;14(9):2732-9. PMCID: PMC2950700. |
| Kolesar JM, Schelman WR, Geiger PG, Holen KD, Traynor AM, Alberti DB, Thomas JP, Chitambar CR, Wilding G, Antholine WE. Electron paramagnetic resonance study of peripheral blood mononuclear cells from patients with refractory solid tumors treated with Triapine. J Inorg Biochem. 2008;102(4):693-8. PMCID: PMC2390869. |
| Flaherty KT, Schiller J, Schuchter LM, Liu G, Tuveson DA, Redlinger M, Lathia C, Xia C, Petrenciuc O, Hingorani SR, Jacobetz MA, Van Belle PA, Elder D, Brose MS, Weber BL, Albertini MR, O’Dwyer PJ. A phase I trial of the oral, multikinase inhibitor sorafenib in combination with carboplatin and paclitaxel. Clin Cancer Res. 2008;14(15):4836-42. |
| Chaiswing L, Zhong W, Cullen JJ, Oberley LW, Oberley TD. Extracellular redox state regulates features associated with prostate cancer cell invasion. Cancer Res. 2008;68(14):5820-6. |
| Attia S, Eickhoff J, Wilding G, McNeel D, Blank J, Ahuja H, Jumonville A, Eastman M, Shevrin D, Glode M, Alberti D, Staab MJ, Horvath D, Straus J, Marnocha R, Liu G. Randomized, double-blinded phase II evaluation of docetaxel with or without doxercalciferol in patients with metastatic, androgen-independent prostate cancer. Clin Cancer Res. 2008;14(8):2437-43. |
| Takimoto CH, Graham MA, Lockwood G, Ng CM, Goetz A, Greenslade D, Remick SC, Sharma S, Mani S, Ramanathan RK, Synold TW, Doroshow JH, Hamilton A, Mulkerin DL, Ivy P, Egorin MJ, Grem JL. Oxaliplatin pharmacokinetics and pharmacodynamics in adult cancer patients with impaired renal function. Clin Cancer Res. 2007;13(16):4832-9. |
| Synold TW, Takimoto CH, Doroshow JH, Gandara D, Mani S, Remick SC, Mulkerin DL, Hamilton A, Sharma S, Ramanathan RK, Lenz HJ, Graham M, Longmate J, Kaufman BM, Ivy P, National Cancer Institute Organ Dysfunction Working Group study. Dose-escalating and pharmacologic study of oxaliplatin in adult cancer patients with impaired hepatic function: a National Cancer Institute Organ Dysfunction Working Group study. Clin Cancer Res. 2007;13(12):3660-6. |
| Zhang D, Pier T, McNeel DG, Wilding G, Friedl A. Effects of a monoclonal anti-alphavbeta3 integrin antibody on blood vessels – a pharmacodynamic study. Invest New Drugs. 2007;25(1):49-55. |
| MacKenzie DA, Schartner J, Lin J, Timmel A, Jennens-Clough M, Fathman CG, Seroogy CM. GRAIL is up-regulated in CD4+ CD25+ T regulatory cells and is sufficient for conversion of T cells to a regulatory phenotype. J Biol Chem. 2007;282(13):9696-702. |