Translational research is often described as having four main areas of translation, referred to as T1 – T4. All the areas cross react with the goal of translating science into high quality care to benefit human health. The process starts with discovery science – bench research:
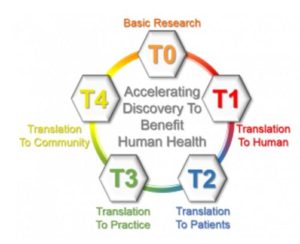
- T1 involves the translation of basic science discoveries from the laboratory to human studies, (bench to bedside) including completion of preclinical studies and development of human clinical trial protocols.
- T2 involves translation to patients in clinical settings to inform evidence-based guidelines.
- T3 involves the translation of new therapies/practices resulting from clinical research studies to clinical practice (bedside to community), including adoption of new therapies/practices in clinics in the community.
- T4 is the translation to population health and policy by population-based outcomes studies in the community.
View Drug Discovery: Preclinical Research Development Flow for a map describing drug development from concept to implementation.
Contact Us
OTRS@uwcarbone.wisc.edu
608-262-8016
Translational Research Resources
The National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health, conducts and supports research on the science and operation of translation to allow more treatments to get to more patients more quickly. Looking for funding for your research? Contact NCATS for more information.
The Process from Basic Science Drug Discovery to Clinical Research
Early Stage Preclinical Development
In this stage, drug discovery is protected for future development, preclinical efficacy is established, and the Investigational New Drug (IND) process is initiated.
This is an accordion element with a series of buttons that open and close related content panels.
Agreements for Materials/Data/Confidentiality
Agreements and protections are needed to ensure successful translation of the research discovery to clinical application in the future. Patent protection is a critical element for successful translation of new drugs to clinical application. You need to ensure you are taking steps to protect your research discovery for future patentability.
OTRS can help guide you through the processing and timely execution of agreements and invention disclosures you may need for your research discoveries. If you need any assistance with an agreement, please contact nonfunded_agreements@uwcarbone.wisc.edu with your request.
Material Transfer Agreement (MTA) for incoming or outgoing research material
- MTAs protect the rights of both parties in the transfer of research materials
Confidential Disclosure Agreement (CDA), also known as Non-Disclosure Agreement (NDA), for protection of confidential information
- Needed for transfer of confidential information between a company and University investigators
- Must be signed by a University official
Intellectual Property Agreement (IPA) for compliance with sponsored research agreement terms and conditions
- A school or college may require staff and students to enter into an IPA
- For disclosure of inventions and compliance with provisions of sponsored research agreements
Invention Disclosure Report (IDR) for equity review and potential protection of your discovery
- All discoveries by UW-Madison faculty, staff or students must be disclosed in an IDR to WARF
- Once the IDR is processed by WARF, the Graduate School will complete an equity review to determine who has ownership rights to the invention
- Following that, the inventor may have the option to work with WARF for patent protection
- IDRs/patent protection ideally should be in place before public disclosure
Sources for Additional Forms and Information
Preclinical Testing Research
Several areas of preclinical testing require completion before a new drug can move into clinical research. The UWCCC Drug Development Core Shared Resource supports world-class biomedical research by facilitating drug development in all therapeutic areas through the integration of high throughput screening, medicinal chemistry and pharmacology. Contact OTRS if you need assistance with finding resources to complete your preclinical testing research.
Preliminary Formulation
- Determine formulation
- Establish route of administration
Bioanalytical Methods
- Methods to test drug/blood/tissue interactions of the intended formulation
Analytical Methods
- Methods to analyze drug characteristics (solubility, pH, stability, and test against safety specifications) of the intended formulation
PK Metabolism
- Show drug bioavailability (orally bioavailable drug is ideal)
- Single dose administration to determine the amount of drug in blood/tissue
- Administration, Dose, Metabolism, Excretion (ADME) in a preclinical model gathered from three different sources (animal model/tissue collection/bioanalytical testing)
Dose Range-Finding
- Establish Maximum Tolerated Dose (MTD)
Efficacy
Establish efficacy against disease model/drug target in vivo
Pre-IND Protocol Development
As preclinical testing research is coming to completion / preclinical efficacy is demonstrated, preparation for an IND application should begin, including IND-required toxicity studies and clinical protocol development.
What is an Investigational New Drug (IND)?
The Investigational New Drug (IND) Application fulfills the following two regulatory requirements:
- Federal law requires that a drug be FDA approved before it can be transported across state lines. The IND is a technical request for exemption from the federal law.
- The IND is a request for permission to conduct clinical trials by supplying relevant non-clinical, CMC and previous human experience (if applicable) in the application.
What is the purpose of an IND?
To assure data quality and study subject safety by requiring the following:
- Animal pharmacology and toxicology data
- Manufacturing information
- Clinical protocols and Investigator information
Types of INDs:
- Investigator IND – submitted by a physician who both initiates and conducts the investigation, and under whose immediate direction the investigational drug is administered or dispensed.
- Emergency Use IND – allows the FDA to authorize use of an experimental drug in an emergency situation that does not allow time for submission of an IND in accordance with 21CFR, Sec 312.23 or Sec. 312.34.
- Treatment IND – submitted for experimental drugs showing promise in clinical testing for serious or immediately life-threatening conditions for which there are no satisfactory alternative treatments. The experimental drug is made available while the final clinical work is conducted and the FDA review takes place.
Pre-IND meeting with FDA:
When
- Novel indication (new use for marketed drug)
- No current Guidance Documents available from the FDA on the new agent
- Unique (new) molecular identity, studies, or indications
- New sponsors or new to area of drug development
Why
- Guidance in Pharm/Tox studies
- Problematic Pharm/Tox studies
- Avoid protocol amendments
Objectives
- Review and reach agreement on the design of animal studies needed to initiate human testing
- Discuss the scope and design of Phase 1 testing, plans for studying the drug product in pediatric populations and best approach for presenting and formatting data in the IND
IND content and format requirements
Contents of IND application (reference 21 CFR 312.23)
- FDA Form 1571
- Table of Contents
- Introductory Statement
- General Investigational Plan
- Investigator’s Brochure
- Protocols
-
- Investigator data
- Facilities data
- IRB data
- Chemistry, Manufacturing and Control data
-
- Environmental assessment or claim for exclusion
- Pharmacology and toxicology data
- Previous human experience
- Additional information to be considered
- FDA IND Application Information
Late Stage Preclinical Development
In this stage, clinical grade drug is produced, toxicity studies required for IND are completed, clinical protocol is developed and IND application is submitted.
This is an accordion element with a series of buttons that open and close related content panels.
Drug Manufacturing
Drug manufacturing provides specific information necessary for the IND submission such as:
- Data which supports the purity, identity, strength and stability under various conditions (such as temperature, light and time)
- Impurity profile consistent with the material used in nonclinical toxicology studies
- Formulation consistent between the drug used in nonclinical studies and clinical formulation
While the intent of drug development is to provide a formulation that provides the appropriate drug delivery characteristics necessary for clinical trials, drug formulation used in Phase 1 clinical trials may simply be an oral solution or a suspension dose which may be different than the final manufactured product.
Pharmacology/Toxicology (Pharm/Tox)
The Pharm/Tox supporting sections of the IND contain an integrated summary of the information obtained from the nonclinical studies. The intent of nonclinical toxicology and pharmacology studies typically performed on large animal models is to:
- Identify a safe starting dose or dose escalating scheme for the proposed clinical trial
- Monitor toxic effects on organs for identification of organ toxicity that should be specifically monitored in the proposed clinical trial
- Evaluate carcinogenicity and teratogenicity risks
Common toxicology testing may include:
- Repeat dose toxicity
- Absorption, distribution, metabolism, and excretion studies (ADME)
- Genotoxicity
- Carcinogenicity
- Immunotoxicity
- Reproductive toxicity
However, the exact toxicology testing required will depend on the intended clinical use.
Additionally, a very limited assessment of safety pharmacology should be performed. Core battery tests should include evaluation of vital organ function: cardiovascular, respiratory, and central nervous system.
Clinical Protocol/IND Submission
OTRS can help make connections for clinical protocol development and the IND process as well as assist with the IND application submission.
Please refer to IND section (pre-IND drop-down, above) for the list of requirements necessary for the IND submission.
In addition to IND application items, the following details for the clinical protocol are extremely important in the submission:
- Starting dose
- Dose escalation
- Inclusion/exclusion criteria for the population to be studied
- Definition of dose-limiting toxicity
- Monitoring of adverse effects
Go/Hold Decision
In this stage, the IND application is reviewed by the FDA, a go or hold decision is rendered. The FDA has 30 days from receipt of the IND to contact the sponsor with issues that need to be resolved before the clinical trial can begin. If the FDA does not contact the sponsor by day 30, it is assumed that there are no problems and the sponsor can proceed with the protocol.
Once the sponsor is notified of issues, the sponsor has 30 days to either:
- Amend the protocol, get FDA approval of the amendment, then proceed with the protocol
- Not amend, and/or the amendment is not approved by the FDA; protocol is placed on clinical hold until all of the issues are addressed