The mission of the Human Cancer Virology Program is to advance the understanding, prevention, and treatment of virus-associated cancers and other cancers through basic research.
Research Areas
Human tumor viruses are causally associated with over 15% of all human cancers and are likely to contribute to additional cancers. Recent research in our program and elsewhere clearly demonstrates that, for many virus-associated cancers, viral gene functions are required both for tumor development and also for continued survival of malignant cells. Thus, inhibiting tumor virus infection, replication and maintenance, and/or selected viral oncogenic functions can prevent or cure many of these cancers. Accordingly, the thematic aims of the UWCCC Human Cancer Virology Program (VR) are:
Thematic Aim 1: To elucidate the pathways by which diverse tumor viruses infect, replicate, interact with their hosts, and maintain their presence through the years to decades typically associated with tumor progression.
Thematic Aim 2: To determine the mechanisms by which tumor viruses promote tumor induction and maintenance, including identifying viral and host genes essential to the continuing survival of tumor cells.
Thematic Aim 3: To define molecular changes and diagnostic/prognostic biomarkers of virally induced infections and neoplasia and use these insights to develop novel, improved approaches to prevent and treat tumor virus infection, replication, tumor induction, and maintenance.
Program Leaders
Robert Kalejta
Eric Johannsen
Human Cancer Virology Membership
View all Human Cancer Virology program members at UWCCC
Select Recent Accomplishments
This is an accordion element with a series of buttons that open and close related content panels.
EBV Z Promoter Polymorphism/Cancer Risk
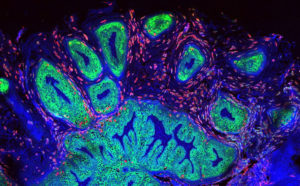 Kenney and Johannsen recently discovered that a polymorphism in the EBV promoter (Zp) controlling lytic EBV reactivation dramatically increases the amount of lytic EBV gene expression in B cells (m.s. submitted). Importantly, this EBV promoter variant (Zp-V3) is over-represented (relative to nonmalignant tissues of EBV+ patients in the same geographic regions) in both nasopharyngeal carcinomas in Hong Kong and AIDS-related lymphomas in Italy. Since this promoter polymorphism is more common in Africa and Asia than in the US, Kenney, Johannsen, and Striker will now collaborate with organ transplant physicians to determine if this Zp-V3 variant is also over-represented in post-transplant lymphoproliferative disease (PTLD) in Wisconsin relative to its frequency in normal individuals. If so, this EBV polymorphism may serve as a biomarker for transplant patients at highest risk for developing EBV-induced PTLD. In this case, this collaboration will next determine if inhibiting EBV lytic infection in transplant patients infected with Zp-V3 EBV strains reduces the risk of EBV-induced PTLD.
Kenney and Johannsen recently discovered that a polymorphism in the EBV promoter (Zp) controlling lytic EBV reactivation dramatically increases the amount of lytic EBV gene expression in B cells (m.s. submitted). Importantly, this EBV promoter variant (Zp-V3) is over-represented (relative to nonmalignant tissues of EBV+ patients in the same geographic regions) in both nasopharyngeal carcinomas in Hong Kong and AIDS-related lymphomas in Italy. Since this promoter polymorphism is more common in Africa and Asia than in the US, Kenney, Johannsen, and Striker will now collaborate with organ transplant physicians to determine if this Zp-V3 variant is also over-represented in post-transplant lymphoproliferative disease (PTLD) in Wisconsin relative to its frequency in normal individuals. If so, this EBV polymorphism may serve as a biomarker for transplant patients at highest risk for developing EBV-induced PTLD. In this case, this collaboration will next determine if inhibiting EBV lytic infection in transplant patients infected with Zp-V3 EBV strains reduces the risk of EBV-induced PTLD.
Human Papillomavirus, Merkel Cell Polyomavirus and their Causal Role in Cancer

HPV causes over 5% of human cancers, including >95% of cervical cancers, a majority of other anogenital cancers, and ~25% of head and neck squamous cell carcinomas. Lambert has used mouse models to show that cervical cancerdevelopment and maintenance require HPV oncogenes E6 and E7, plus estrogen. Recent work in his laboratory shows that the HPV E6 oncogene contributes to maintenance of HPV genomic DNA in cells both through p53 inactivation and other functions. Additionally, Lambert and Kimple (IR) demonstrated that HPV oncogene E7 delays DNA damage repair responses, potentially explaining the clinically observed greater radiation sensitivity of HPV-positive relative to HPV-negative head and neck cancers, and providing further rationale for radiation de-escalation in treating patients with HPV-positive head and neck cancers.
EBV and KSHV genome replication and segregation studies
Genetic elements that replicate extrachromosomally are rare in mammals, but several human tumor viruses, including the papillomaviruses and gamma-herpesviruses, maintain their plasmid genomes by tethering them to cellular chromosomes. Sugden developed two complementary approaches to uncover an unprecedented clustering mechanism by which Kaposi’s Sarcoma-associated Herpesvirus (KSHV) maintains its genome extrachromosomally: Live-cell imaging and a predictive computational model revealed the formation and dynamic evolution of these clusters over multiple cell generations. This clustering, mediated by KSHV protein LANA1, controls KSHV DNA synthesis, partitioning and copy numbers and thus is central to KSHV survival and oncogenesis.
