The Genetic and Epigenetic Mechanisms (GEM) pursues basic and translational research to discover novel cancer genetic and epigenetic mechanisms, to translate mechanistic insights derived principally from mouse models and primary human cancer samples to human cancer, and to leverage the mechanistic insights to advance cancer diagnosis and treatment. GEM investigators develop new technologies to better detect variants, deploy mechanistic studies to discover the functional consequences of variants and engage in translational efforts to exploit these variants for better prevention, early detection and therapy.
Research Areas
As genetic and epigenetic mechanisms underlie human cancer initiation and progression, components of these mechanisms represent extremely promising targets for cancer prevention, diagnosis, and therapy. Considering the multitude of regulatory factors involved, and the many unanswered mechanistic questions, our vision is that much of the mechanistic knowledge and druggable space remain to be discovered. Thus, from both fundamental and clinical/translational perspectives, elucidating cancer genetic and epigenetic mechanisms continues to hold great promise.
Thematic Aim 1: Discover and Elucidate Cancer Genetic Mechanisms. GEM has made major progress in discovering and dissecting genetic mechanisms with human clinical cancer relevance. Multidisciplinary teams analyze breast, prostate, myeloid, ovarian, and other cancer mechanisms, and use insights to forge clinical trials to improve cancer prevention, diagnosis, and therapy.
Thematic Aim 2: Discover and Elucidate Cancer Epigenetic Mechanisms. Since epigenetic regulators often require particular genetic sequences to act at specific chromatin sites, genetic and epigenetic mechanisms are intertwined. It is instructive to subdivide GEM genetic and epigenetic efforts into distinct themes. GEM discovers and dissects epigenetic mechanisms underlying acquisition and maintenance of malignant phenotypes. Such mechanisms can be broadly applicable, restricted to select cancers and/or operate in the tumor microenvironment.
Genetic & Epigenetic Mechanisms Membership
View all Genetic & Epigenetic Mechnisms program members at UWCCC
SELECT RECENT ACCOMPLISHMENTS
This is an accordion element with a series of buttons that open and close related content panels.
Precision Medicine Paradigm to Increase Chemotherapeutic Efficacy in Breast Cancer
Through analyzing mitotic mechanisms, the Weaver/Burkard team published a landmark paper that led to a paradigm shift in understanding the mechanism underlying efficacy of the chemotherapeutic agent paclitaxel. Whereas paclitaxel is one of the most commonly used drugs to treat primary and metastatic breast cancer, only 41-58% of patients’ benefit. There is no way to predict responsiveness prior to therapy. Non-responsive patients can be subjected to serious side effects, including life-threatening leukopenia and peripheral neuropathy that greatly diminish quality of life and consequent delay in receiving efficacious therapies. A biomarker to identify responsive patients would improve outcomes for many of the 1.7 million patients worldwide that are diagnosed with breast cancer each year.
Multi-parametric Breast Cancer Diagnosis

Using novel machine learning, Page and Burnside are collaborating to improve the identification of patients that require biopsy by using mammography features (variables), electronic health records (EHR), and genetic markers (SNPs) to personalize breast cancer risk prediction and reduce over-diagnosis. The machine learning methods developed show potential to more accurately select women who need interventional procedures, thus sparing those who do not.
Protein Arginine Methyltransferase Function in Breast Cancer
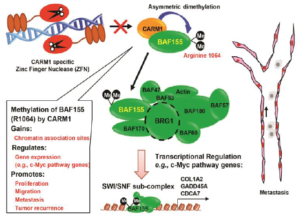
CARM1 encodes a protein arginine methyltransferase that plays a central role in estrogenreceptor (ER)-mediated transcription and breast cancer cell proliferation. These actions involve methylation of histone H3 and the BAF155 subunit of the SWI/SNF chromatin remodeling complex. Xu’s group discovered a reader complex PAF1c that recognizes H3 di-methylated at R17 and demonstrated that the PAF1c subunit Ctr9 is a key player in ER regulons. This study provided rationale for targeting Ctr9 to overcome endocrine resistance in ER-positive breast cancers. Identifying CARM1 substrates will inform cancer mechanisms from diverse perspectives, including understanding drug resistance and establishing how chromatin remodeling controls progression.