Statistical consultation is especially valuable during the planning stage of a research study or a proposal for extramural funding. The early and continuing participation of a biostatistician always improves a grant proposal and the ensuing research. Interim analyses are valuable elements of progress reports, and aid investigators in adjusting the course of their research. The collaboration of biostatisticians in the final analyses, resulting in abstracts for presentation and publications, greatly strengthens the credibility of the study findings to the cancer research community.
Basic Science
Biostatistician: Michael Newton, PhD – Genetics and Genomics, Statistical Computing, Lab Sciences
Collaborators: UWCCC Drug Development Core (DDC), Anthony Gitter, PhD (GEM)
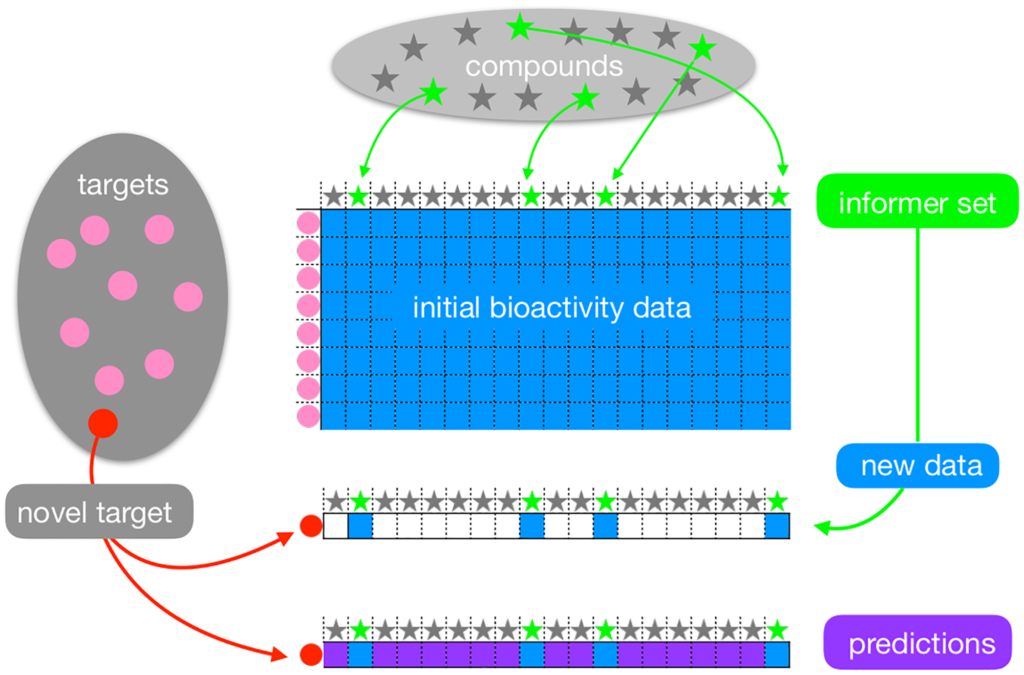
Informer-based ranking (IBR) method selects an informer set of compounds, and then prioritizes the remaining compounds on the basis of new bioactivity experiments performed with the informer set on the target. We formalize the problem as a two-stage decision problem and introduce the Bayes Optimal Informer SEt (BOISE) method for its solution.
One NIH methodology grant (R01GM135631, PI: Gitter (GEM) and Newton (GEM)) was awarded to develop a computational platform.
Clinical Science
Biostatisticians: Kyungmann Kim, PhD – Clinical Trials, Clinical and Translational Studies
Collaborators: – Paul Sondel, MD, PhD (DT)
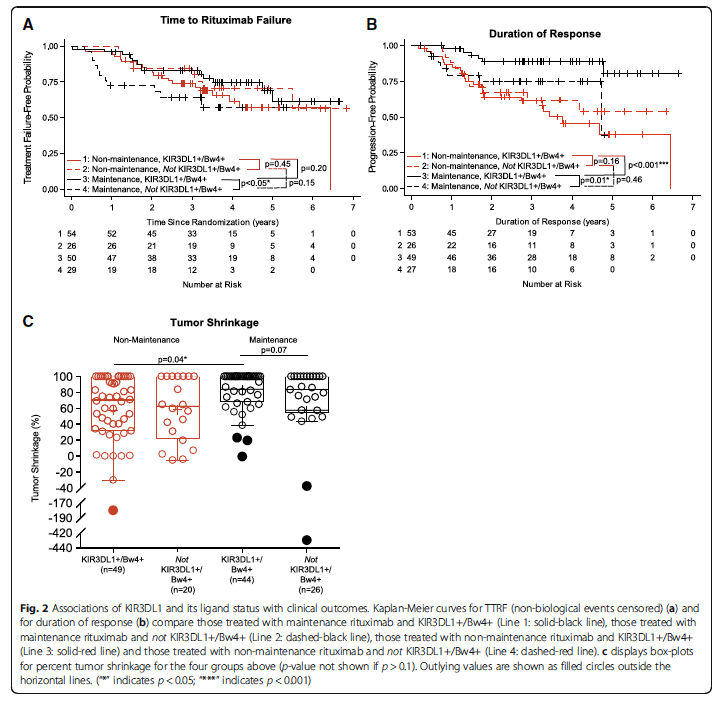
For patients with low-tumor-burden FL, a maintenance rituximab therapy has improved progression free survival. Yet, whether other clinical outcomes could benefit from a continual ‘maintenance’ schedule (rituximab every 13 weeks) vs. a ‘non-maintenance’ (no additional rituximab until progression) treatment was unclear.
BSR supported Dr. Sondel and colleagues in determining whether inherited genotypic variances in genes that influence immune function may identify subpopulations of FL patients that differ in their outcome following maintenance vs. non-maintenance schedules. Dr. Kim (CPC & BSR) lead the statistical analysis to assess association of genotypes for KIR/KIR-ligands with clinical outcomes as a post-hoc analysis of a ECOG-ACRIN phase III trial which evaluated rituximab treatment schedules for FL patients.
Population-Based Science
Biostatistician: Ron Gangnon, PhD – Spatial Statistics, Cancer Prevention and Control, Epidemiology
Collaborators: Amy Trentham-Dietz, PhD (CPC)
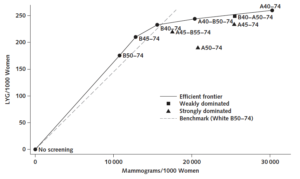
Current screening mammography guidelines do not consider racial differences in breast cancer epidemiology, treatment, and survival. Black women may need different screening schedules to achieve similar screening outcomes to White women.
Drs. Gangnon (CPC & BSR) and Trentham-Dietz (CPC) contributed to the first study using simulation modeling to consider whether race-neutral guidelines for breast cancer screening lead to unequal outcomes.
Results suggest that, in self-identified Black women, initiation of earlier screening can reduce mortality disparities and maintain acceptable benefit–harm tradeoffs.
Past Publications
This is an accordion element with a series of buttons that open and close related content panels.
Basic Science
Biostatistician: Michael A. Newton, PhD – Cancer Genetics Program
Collaborators: Paul Ahlquist, PhD and Paul F. Lambert, PhD – Human Cancer Virology Program
I/IIa clinical trial in 22 stage D0 prostate cancer patients was conducted to evaluate the safety of a DNA-based vaccine encoding Prostatic Acid Phosphatase (PAP).
Working with Drs. Lambert and Ahlquist, Dr. Newton analyzed whole-genome profiles from human tissue samples. Findings provided novel biomarkers for early detection and emphasized the potential value of targeting E6 and E7 function in the treatment of HPV+ cancers.
Clinical Science
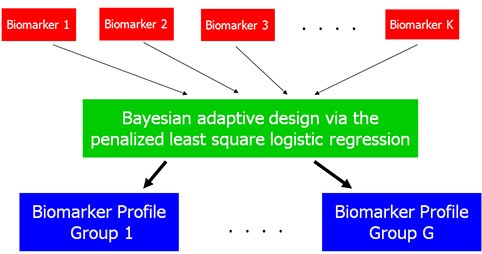
Biostatisticians: Jens C. Eickhoff, PhD – Experimental Therapeutics Program; KyungMann Kim, PhD – Chemoprevention Program
Collaborators: Jill M. Kolesar, PharmD – Experimental Therapeutics Program; Howard H. Bailey, MD – Chemoprevention Program
Motivated by their collaboration with Drs. Kolesar and Bailey, Drs. Kim and Eickhoff developed optimal clinical trial designs for pharmacogenomics-driven targeted therapies that directly integrated information about biomarkers and clinical outcomes as they become available. The design efficiently identified patients who benefit most from a targeted therapy. There were substantial savings in the sample size requirements when compared to alternative designs.
Population-Based Science
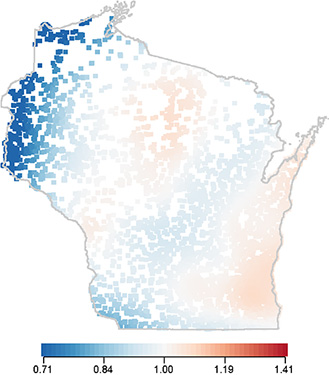
Biostatistician: Ronald E. Gangnon, PhD – Cancer Control Program
Collaborators: Amy Trentham-Dietz, PhD, Jane McElroy, PhD, Patrick Remington, PhD, and Polly A. Newcomb, PhD – Cancer Control Program
Using geocoded residential locations for case-control study participants, Dr. Gangnon utilized a generalized additive logistic regression model to estimate geographic risk as a local odds ratio using a two-dimensional thin plate spline while adjusting for established risk factors. Results suggest that established breast cancer risk factors do not explain long-standing observations of higher breast cancer mortality in eastern WI counties.